Experimental
Methyl Chloroacetate
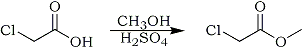
94.5g (1 mole) of chloroacetic acid was dissolved in 350ml (10 moles) of methanol, 2.5ml (5g) of 98% H2SO4 was added, and the mixture was refluxed for 12 hours. The solution was neutralized with Na2CO3, filtered, and most of the methanol was removed by evaporation. The residue was washed with 100ml of water, dried over Na2SO4 and distilled, pure methyl chloroacetate was collected at 130-132°C. (Caution: Methyl Chloroacetate is a lachrymator!)
Methyl 2,5-Dimethoxyphenylglycidate
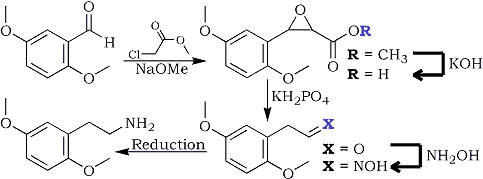
A 15% solution of sodium methoxide in methanol was prepared by dissolving 2.75 g freshly cut sodium metal (120 mmol) in 50 mL of MeOH (Caution: Strongly exothermic, slowly add small pieces). The resulting solution is then added dropwise during one hour to a well-stirred solution of methyl chloroacetate (140 mmol, 10.9g) in which 2,5-dimethoxybenzaldehyde (100 mmol, 16.6g) had been dissolved. Throughout the addition, the temperature was kept between 15-20°C, after which time the stirring was continued for another hour at room temperature.
2,5-Dimethoxyphenylglycidic acid
During two hours, the above ester solution is added dropwise to a solution of 9.8g 85% KOH (0.15 moles) in 13ml water, keeping the temperature between 15-20°C. The reaction mixture is stirred for three more hours at this temp, then at room temp for 12 hours, then cooled to 10°C and filtered.
2,5-Dimethoxyphenylacetaldehyde
The solid is washed with 2x15ml of methanol, and subsequently it is added to a mixture consisting of 50ml water, 50ml methylene chloride and 14g KH2PO4, while keeping the reaction mixture under stirring for two hours at room temp. The two layers are then separated, the aqueous phase is extracted twice with 25ml methylene chloride, then discarded, while the organic layers are collected and washed with 25ml of dH2O.
2,5-Dimethoxyphenylacetaldoxime
The pooled organic extracts are added dropwise with strong stirring into a 25% aqueous solution of 0.3 moles hydroxylamine (from 20.9 g hydroxylamine hydrochloride and 12g NaOH in 100 ml water) while keeping the temperature at -5°C for about two hours. Then 0.4 grams of sodium chloride is added, and the layers are separated, the aqueous layer is extracted with 3x10ml of DCM, and the pooled organic extracts are added to 50ml of methanol.
The above aldoxime is then reduced using any suitable method to yield 2,5-Dimethoxyphenethylamine (2C-H).