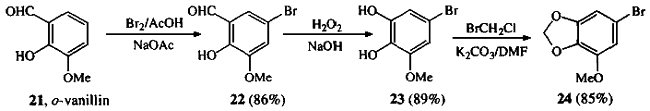
Treatment of o-vanillin 21 with bromine in a mixture of sodium acetate and glacial acetic acid gave 5-Bromo-3-methoxysalicylaldehyde 221, which was subjected to Dakin oxidation conditions to give 3,4-Dihydroxy-5-methoxybromobenzene 232. Methylenation of 23 gave the known 3,4-Methylenedioxy-5-methoxybromobenzene 243.
Experimental
5-Bromo-3-methoxysalicylaldehyde (22)
To a solution of 21 (73.7 g, 0.48 mmol) and sodium acetate (60 g, 0.73 mmol) in acetic acid (1.8 L) was added bromine (25 mL, 0.48 mmol) in acetic acid (20 mL). The resultant yellow solution was stirred at room temperature for 30 min. Acetic acid was evaporated in vacuo, and the residue was partitioned between CH2Cl2 (1 L) and H2O (1 L). The organic phase was washed with H2O (2x1 L), dried (MgSO4) and evaporated in vacuo to leave a brown solid. Recrystallization from acetic acid/H2O gave 22 (96.2 g, 0.416 mol, 86%) as yellow needles, Rf 0.53 (CH2Cl2/hexanes 4:1); mp 122-124°C (lit1 128-129°C).
3,4-Dihydroxy-5-methoxybromobenzene (23)
To a solution of 22 (20 g, 86.6 mmol) in 2% NaOH (425 mL) at room temperature was added a solution of 30% H2O2 (54 g, 476 mmol) in H2O (510 mL). The mixture turned a deep purple color and was left to stir for 30 min; 2M HCl (50 mL) was added and the solution lightened to a pale pink color. The aqueous solution was extracted with CH2Cl2 (4x250 mL). The combined extracts were washed with saturated aqueous Na2SO3 (2x500 mL), and dried (Na2SO4). Evaporation in vacuo gave crude 23 (16.8 g, 77 mmol, 89%) as a white solid, Rf 0.28 (hexanes/EtOAc 7:3); mp 72-74°C (lit2 74-76°C).
3,4-Methylenedioxy-5-methoxybromobenzene (24)
To a mixture of 23 (20 g, 90.9 mmol) in DMF (230 mL) and K2CO3 (18.9 g, 136 mmol) was added bromochloromethane (6.6 mL, 100 mmol). The mixture was warmed to 80°C for 2 h during which time it turned a dirty green color. A further portion of bromochloromethane (6.6 mL, 100 mmol) was added and heating continued for 1 h. The solution was allowed to cool, diluted with H2O (230 mL) and extracted with Et2O (3x250 mL). The combined ethereal layers were washed with brine (200 ml) and dried (MgSO4). Evaporation in vacuo yielded crude product, which was purified by chromatography over silica gel eluting with hexanes/Et2O (9:1). Recrystallization from Et2O/hexanes gave 24 (17.67 g, 76.5 mmol, 85%) as white crystals, Rf 0.66 (hexanes/Et2O 4:1); mp 80-81°C (lit3 78-79°C).