Experimental
5-methyl-1,3-benzodioxole (3,4-Methylenedioxytoluene)
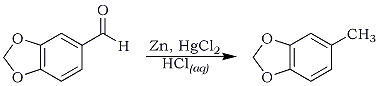
A 1000 mL round-bottomed flask immersed in an efficient cooling system was charged with 20 g of mossy zinc, 12 g of mercuric chloride, 200 mL of water and 6 mL of concentrated hydrochloric acid. An exotherm was detected when shaking the mixture for 5 min. After decanting the liquid layer, 100 mL of benzene and 50 g of piperonal were carefully added to the amalgamated zinc remaining in the flask. The flask was immersed in a heating mantle and the mixture was vigorously refluxed for 24 hr. After cooling the mixture to 25°C and separating the benzene layer, the aqueous layer was extracted with two 75 ml, aliquots of ether. The benzene and the etherate extracts were combined and dried over anhydrous calcium chloride for 24 hr. The solvents were evaporated under reduced pressure. The residue was distilled to yield 14.4 g, 32%, of 5-methyl-1,3-benzodioxole, 85°C/26 mmHg.
5-Acetyl-1,3-benzodioxole (3,4-Methylenedioxyacetophenone, Acetopiperone)
On mixing 15 g (0.123 mol) of 1,3-benzodioxole, 14.2 g (0.123 mol) of trifluoroacetic acid and 27 g (0.265 mol) of acetic anhydride, a yellow color was observed. The mixture turned black upon heating for 2 hr at 65-70°C. Heating was continued for an additional 7 hr. To neutralize the excess of acid, the organic portion was washed with 10% sodium carbonate and water. Unreacted acetic anhydride and 1,3-benzodioxole were removed by vacuum distillation (90°C/60 mmHg) and 10.4 g of a red substance was obtained. This red residue solidified upon refrigeration. Recrystallization in hot ethanol afforded a shiny brown powder, m.p 82-83°C.
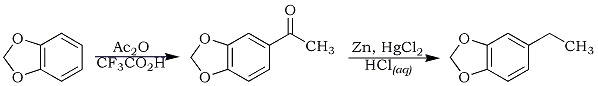
5-Ethyl-1,3-benzodioxole
This was performed through Clemmensen reduction of 5-acetyl-1,3-benzodioxole, using the amalgamated zinc recovered from the reduction of piperonal. A similar procedure was also employed using 5 g of 3,4-methylenedioxyacetone and adjusting the amounts of reagents used. The distillation under reduced pressure yielded 2 g, 44%, of colorless 5-ethyl-1,3-benzodioxole, bp 82°C/25mmHg.