Summary
The recent identification of the anorectic agent 4-methylaminorex (2-amino-4-methyl-5-phenyl-2-oxazoline) as a police exhibit in Canada led to the need to synthesize a reference standard specimen. Since this substance can occur as cis- and trans- isomers, both had to be prepared and characterized spectrally. cis-4-Methylaminorex was made from the erythro amino alcohol norephedrine (phenylpropanolamine), while the trans compound was made from the threo diastereoisomer norpseudoephedrine, in both cases by the treatment of the substrate with cyanogen bromide in a methanolic solution of sodium acetate. In this paper, the 13C- and 1H-NMR, infrared (IR), mass (MS) and ultraviolet (UV) spectra are reported, together with a commentary on the distinguishing features noted between the sets of spectra.
Introduction
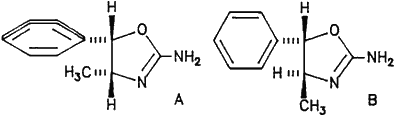
Fig. 1.
cis-4-methylaminorex (A) and trans-4-methylaminorex (B)
Several samples of 4-methylaminorex were received in gram quantities as police exhibits in our laboratory during 1987. The substance was also reported in the United States1, where it was described as a potent central nervous system stimulant. Scheduling action was taken in both Canada and the U.S., since the substance had not previously been controlled. Although originally synthesized as a potential anorectic agent2, it was later investigated for its hypertensive and central nervous system stimulating activities3,4. 4-Methylaminorex can occur in both a cis- and a trans- form (Fig. 1) and it is important to be able to characterise these. Both isomers have therefore been synthesized and their spectroscopic properties measured.
Experimental
Both specimens used in this study were synthesized according to the procedure of Poos et al.2 with yields and melting points as reported.
Spectra were obtained on the following instruments:
- 13C and 1H-NMR on a Bruker WP-80 transform spectrometer at 20.1 and 80 MHz
respectively, in CDCl3 using Me4Si as internal standard (Fig. 2 and Fig. 3). - IR spectra were obtained on a Nicolet 60SX Fourier spectrometer, as KBr dispersions (Fig. 4).
- Mass spectra were obtained on a Finnigan Mat model 4610B instrument (Fig. 5).
- UV spectra were obtained on a Varian DMS-90 UV-VIS spectrometer (Fig. 6).
Discussion
Table 1
Chemical Shifts in the NMR Spectra of cis- and
trans- 4-Methylaminorex and their Assignments
|
Cis
|
Trans
| ||
|
δ13C (ppm)
|
C no.a
|
δ13C (ppm)
|
C no.
|
|
160.6
|
2
|
159.9
|
2
|
|
137.6
|
1'
|
140.4
|
1'
|
|
128.5
|
(3')
|
128.9
|
(3')
|
|
128.0
|
4'
|
128.5
|
4'
|
|
126.3
|
(2')
|
126.0
|
(2')
|
|
84.7
|
5
|
89.2
|
5
|
|
63.0
|
4
|
68.5
|
4
|
|
18.5
|
CH3
|
21.6
|
CH3
|
|
δ1H (ppm)b
|
H
|
δ1H (ppm)
|
H
|
|
7.4 (m)
|
2',3',4'
|
7.3 (m)
|
2',3',4'
|
|
5.6 (d, J4,5=8.8)
|
5
|
4.9 (d, J4,5=7.3)
|
5
|
|
5.0 (s, broad)
|
NH2
|
4.4 (s, broad)
|
NH2
|
|
4.3 (m, J4,CH3=7.0)
|
4
|
4.0 (m, J4,CH3=6.5)
|
4
|
|
0.7 (d)
|
CH3
|
1.3 (d)
|
CH3
|
- Values in brackets may be reversed
- s, singlet; d, doublet; m, multiplet
NMR: The main differences in chemical shifts between the cis and trans isomers (Table 1) are due to the steric interactions between the methyl and phenyl groups in the cis compound. Thus, in the 13C spectra, the chemical shifts for C1', C4, C5 and the CH3 are all ~3-5 ppm to higher field in the cis isomer. In the 1H spectra, H5 for the cis isomer is 0.7 ppm to lower field while the CH3 is 0.6 ppm to higher field. The cis methyl protons are shifted to higher field because they are in the shielding region of the phenyl ring.
IR: As expected, there are significant differences in the fingerprint region of the respective IR spectra. In addition, both isomers show a strong, narrow band at 3442 cm-1 (trans) and 3423 cm-1 (cis) due to the NH2 vibration.
MS: The two mass spectra (Figs. 5A and B) are virtually indistinguishable, as expected. The base peak in each case is at m/z 70, and there is a prominent molecular ion at m/z 176.
UV: The two UV spectra (Figs. 6A and B) are superimposable, with a maximum at 258 nm, and an E1%1cm of 11.6. From an examination of the spectra published in this report, it is clear that neither the MS nor the UV data could be used to assist in making a spectral distinction between the two compounds. A comparison of spectra obtained from the two synthesized standards with those from the sample seized by the police showed that the seized material was the cis-isomer.