4-Cyanophenylhydrazine HCl1
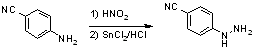
To a cooled (-15°C) and stirred suspension of 4-aminobenzonitrile (50g, 423 mmol) in concentrated hydrochloric acid (550 ml) was added dropwise a solution of sodium nitrite (31.5g, 457 mmol) in water (200 ml) at such a rate as to maintain the temperature below -10°C. After the addition was finished, the reaction mixture was quickly filtered to remove solids and the filtrate was added portionwise to a cooled (-20°C) and stirred solution of SnCl2·2 H2O (477 g, 2.1mol) in 370ml conc HCl at such a rate as to maintain the temperature below -10°C. After further 15 min at -10°C to 0°C, the white precipitate was collected by filtration, washed with ether (4x250 ml) and dried to give 56 g (78%) of the title compound; mp 235-237°C. (EtOH/H2O 1:1).
5-Cyanotryptamine HCl1
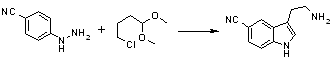
To a stirred suspension of 4-cyanophenylhydrazine HCl (50 g) in a mixture of ethanol and water (5:1; 2L) was added 4-chlorobutanal dimethylacetal (45g) and the resulting mixture was refluxed for 18h. Solvents were removed under vacuum and the residue was azeotroped with toluene to give a brown solid. Crystallisation of this crude material from MeOH (150 ml) gave 23g (35%) of the title compound as a yellow solid; mp 270-274°C.
5-Cyano-N,N-dimethyltryptamine (5-CN-DMT)1
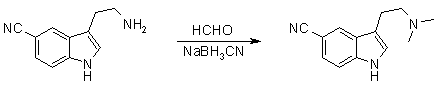
To a cooled (5°C) and stirred solution of sodium methoxide in anhydrous methanol (from 1.75 g of sodium metal in 400 ml of methanol) was added 5-cyanotryptamine HCl (18.6 g) and the mixture was stirred for a further 5 min before sodium cyanoborohydride (7.2 g) and glacial acetic acid (10.4 ml) were added. A solution of formaldehyde (37% aqueous solution; 19.9 ml) in methanol (50 ml) was added dropwise over 30 minutes and the mixture was then allowed to warm to room temp and stirred for a further 15 min. Solvents were removed under vacuum and the residue was diluted with saturated aqueous potassium carbonate (400 ml) and products were extracted with ethyl acetate (3x400 ml). The combined organic extracts were dried (MgSO4) and concentrated. Flash chromatography of the residue (silica gel, dichloromethane-methanol-ammonia, 40:8:1) gave 10.5 g of the title compound as a pale yellow solid.
5-Cyano-N,N-dimethyltryptamine (5-CN-DMT)2
0.121g (0.545 mmol) 5-cyanotryptamine was dissolved in 25ml methanol, and with good stirring 0.1ml 30% methanolic sodium methoxide (1 eq) was added, followed by 0.125ml HOAc (2.18 mmol) and 68mg (1.09 mmol) NaBH3CN. Next 0.103ml 38% aqueous formaldehyde in 10ml methanol was added over 30 minutes. The solution was allowed to stir for 3.5h at room temperature, and 25ml 4M NaOH was added and the methanol evaporated off under vacuum. The residue was extracted with 3x25ml Et2O, the organic extracts washed with 2x20ml brine, dried over MgSO4 and the solvent evaporated to give 5-Cyano-DMT in 75% yield.