Myristicinaldehyde (3-methoxy-4,5-methylenedioxybenzaldehyde) and 3,4,5-trimethoxybenzaldehyde are intermediates in the synthesis of numerous alkaloids and pharmacological agents. As a part of our studies on the synthesis and pharmacological action of ring-substituted phenethylamines, we had need of a general method for the preparation of the 3-alkoxy homologs of these two aldehydes. The published syntheses of myristicinaldehyde generally utilized myristicin (3-methoxy-4,5-methylenedioxyallylbenzene) as a starting material, which is readily available only from botanical sources; 3,4,5-trimethoxybenzaldehyde is usually prepared by the methylation and reduction of gallic acid. Consequently, the 3-alkyl homologs are not readily synthesized from these natural products and have not been reported in the literature.
The simplest approach to these homologs would be the alkylation of the corresponding hydroxybenzaldehydes, 3; however, 3-hydroxy-4,5-dimethoxybenzaldehyde has been prepared only by a multi-step procedure starting from gallic acid1, and 3-hydroxy-4,5-methylenedioxybenzaldehyde is unknown. Initially we attempted synthesis of these hydroxybenzaldehydes via copper catalyzed displacement of bromine or iodine by hydroxide from the corresponding halo-substituted aldehydes 1. None of the expected products were obtained, although the analogous transformations of the related compounds 5-bromovanillin and 5-iodovanillin have been reported2.
Consequently, we have developed an indirect procedure for the replacement of a bromine with a hydroxyl group in the readily available bromobenzaldehydes 1. These can be converted to the corresponding 5-hydroxy counterparts 3 in good to excellent yields through lithium-halogen exchange3 of the cyclohexylamine adducts 24, conversion of the lithio derivatives to organoboranes with B(OBu)35, oxidation of the C-B bond with basic hydrogen peroxide6, and finally, hydrolysis of the protecting Schiff's base.
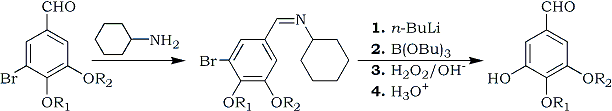
(a): R1 = R2 = Me (b): R1 = Et, R2 = Me (c): R1, R2 = -CH2-
In the case of the title compound 3c the structure was verified by conversion to known myristicinaldehyde; with 3a and 3b ethylation and methylation of each, respectively, yielded exclusively 3,4-dimethoxy-5-ethoxybenzaldehyde, establishing that there had been neither lithium migration nor dealkylation in the synthetic procedure.
Experimental
Preparation of Schiff's Base Derivatives10
3-Bromo-N-cyclohexyl-4,5-methylenedioxybenzylidineimine (2c)
A mixture of 3-bromo-4,5-methylenedioxybenzaldehyde (1c, 2.2 g)7 and cyclohexylamine (3.6 g) in a distillation flask, was heated with a flame until solution was effected. (ca. 1250) The resulting mixture was distilled in a Kugelrohr apparatus (120-125°C/0.2 mm) to provide 2.4 g of distillate that spontaneously crystallized in the receiver (mp 86-96°C, 80%). An analytical sample was obtained as white crystals from methanol (5 ml/g) with mp 97.5-98.5°C.
2a was prepared similarly from 1a8 yielding a nearly colorless viscous oil, bp 145-155°C/0.1 mm, yield 96%.
2b was prepared similarly from 1b9 yielding an off-white solid, bp 148-155°C/0.5 mm, mp 66-68.5°C, yield 94%. An analytical sample was obtained from methanol (5ml/g) with mp 67-68.5°C.
Preparation of Hydroxyaldehydes
3-Hydroxy-4,5-methylenedioxybenzaldehyde (3c)
In a flask equipped with a magnetic stirrer and protected from air and moisture by means of a helium atmosphere, there was added a solution of 2c (2.2 g, 7.1 mmol) in anhydrous ether (50 ml). The solution was stirred and cooled with an external dry-ice acetone bath resulting in the formation of a light, white crystalline suspension. There was added butyllithium in hexane (5.2 ml of a 1.55 M solution, 8.1 mmol) over a 2 min. period, and with continued stirring the solids dissolved, resulting in a pale yellow solution. While still at -78°C there was added B(OBu)3 (4 ml, 15 mmol) and the stirred solution was allowed to come to room temperature. The reaction was then quenched with saturated aqueous (NH4)2SO4 (20 ml). The ether layer was separated, washed with saturated (NH4)2SO4 (20 ml), and evaporated in vacuo. The residual oil was dissolved in 50% methanol (100 ml) and treated with 30% H2O2 (2 ml). After 15 min. there was added (NH4)2SO4 and water (10 g in 50 ml) resulting in a solution of pH 8. This was extracted with CH2Cl2 (2 x 50 ml), and the pooled extracts evaporated in vacuo. The resulting oil was treated with dilute HCl and heated on the steam bath, effecting complete solution. After cooling, the solution was extracted with CH2Cl2 (2 x 50 ml) and the pooled extracts extracted with 5% NaOH (2 x 50 ml). The aqueous extracts were acidified with HCl, reextracted with CH2Cl2, and the organic extracts evaporated to yield the product as an oil. Distillation at 140-150°C/0.25 mm yielded 3c which crystallized in the receiver. Recrystallization from toluene (40ml/g) yielded an off-white solid (mp 134-134.5°C, 39% yield).
3a was prepared similarly from 2a, yielding a white solid (from toluene/hexane) with mp 64-65°C, yield 64% (lit. mp 60-61°C1a, 70-72°C1b). The intermediate phenolic Schiff's base was obtained as a solid, and a small sample recrystallized from methanol had mp 148-149°C.
3b was prepared similarly from 2b, yielding an off-white solid (bp 110-118°C/0.2 mm, mp 75-77.5°C, yield, 77%). An analytical sample was obtained as a white crystalline solid from cyclohexane (100 ml/g) with mp 77-78°C. The intermediate phenolic Schiff's base was obtained as a solid, and on recrystallization from methanol had a mp 145-146°C.
Verification of Structures of 3a and 3b
3-Ethoxy-4,5-dimethoxybenzaldehyde
To a solution of 3b (7.3 g) in dry acetone (100 ml) there was added CH3I (5 ml) and powdered anhydrous K2CO3 (8 g). The suspension was held at reflux for 6 hrs., the volatiles removed in vacuo, the residue dissolved in water, made strongly basic with NaOH and extracted with CH2Cl2 (3 x 50 ml). The extracts were pooled, washed with dilute NaOH, evaporated in vacuo, and the resulting amber-colored oil distilled (110-120°C/0.4 mm) to yield 7.3 g product that spontaneously crystallized, mp 49-49.5°C, yield 93% (lit. mp 48-50°C11).
Similarly, 3a with ethyl iodide yielded a product (bp 110-118°C/0.25 mm) mp, 48.5-49.5°C (mmp with the above sample, 48.5-49.5°C).
3-Methoxy-4,5-methylenedioxybenzaldehyde (Myristicinaldehyde)
Methylation of 3c according to the procedure above yielded off-white needles, mp 133-134°C. Recrystallization from hexane gave an off-white crystalline solid, mp 134-135°C, mmp with an authentic sample12 (mp 133.5-134.5°C), 133.5-134.5°C, mmp with the starting phenol 3c (mp 134-134.5°C) was 100-108°C.