Total Synthesis of Morphine- and Hasubanane Alkaloids
Johann Mulzera, Jan W. Batsb, Stefanie Porthb, Till Opatzb and Dirk Traunera
a) Institut für Organische Chemie der Universität Wien, Währingerstrasse 38, A-1090 Vienna, Austria. E-mail: (Dirk Trauner) dixi@felix.orc.univie.ac.at
b) Institut für Organische Chemie der Johann Wolfgang Goethe-Universität, Marie-Curie-Strasse 11, D-60439 Frankfurt am Main, Germany.
Received: 3 September 1997 / Uploaded: 3 September 1997
1. Introduction
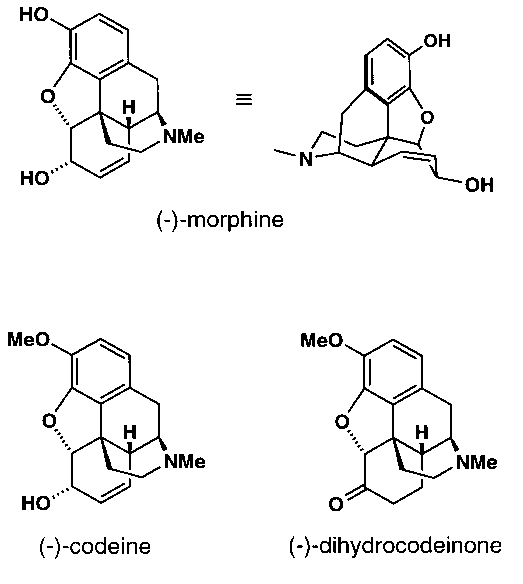
(-)-Morphine, the main alkaloid of the opium poppy, remains one of the most important medicines for the treatment of severe pain and a true delight for synthetic chemists.1 In spite of its small size and long history, it is still considered to be an interesting target for chemical synthesis. (-)-Morphine and its synthetic precursors (-)-codeine and (-)-dihydrocodeinone feature a complicated network of three carbocycles and two heterocycles containing five vicinal stereocenters, four of which define ring junctures. Among these, the benzylic quaternary carbon deserves special attention.

2. Formal Total Synthesis of (-)-Morphine by Cuprate Conjugate Addition2
Recently, we have reported an asymmetric formal total synthesis of (-)-codeine and (-)-morphine which features the conjugate addition of a simple vinyl cuprate to the optically pure enone 1, followed by enolate trapping with N-bromosuccinimide (NBS) (Scheme 1). The X-ray crystal structure of the resultant bromoketone 3 is shown in Figure 1. Due to its conformational preorientation, 3 underwent particularly facile SN2 ring-closure with concomitant demethylation to afford the tetracyclic ketone 3. Ketone 3 was then transformed to sulfonamide 4 which was dehydrogenated by benzylic bromination / dehydrobromination to furnish the styrene 5 (Figure 2). On reductive desulfonation, 5 underwent radical (or anionic) ring-closure to provide the ethylene ketal 6 (Figure 2). Deprotection of 6 finally yielded dihydrocodeinone, a standard synthetic precursor of the opium alkaloids.
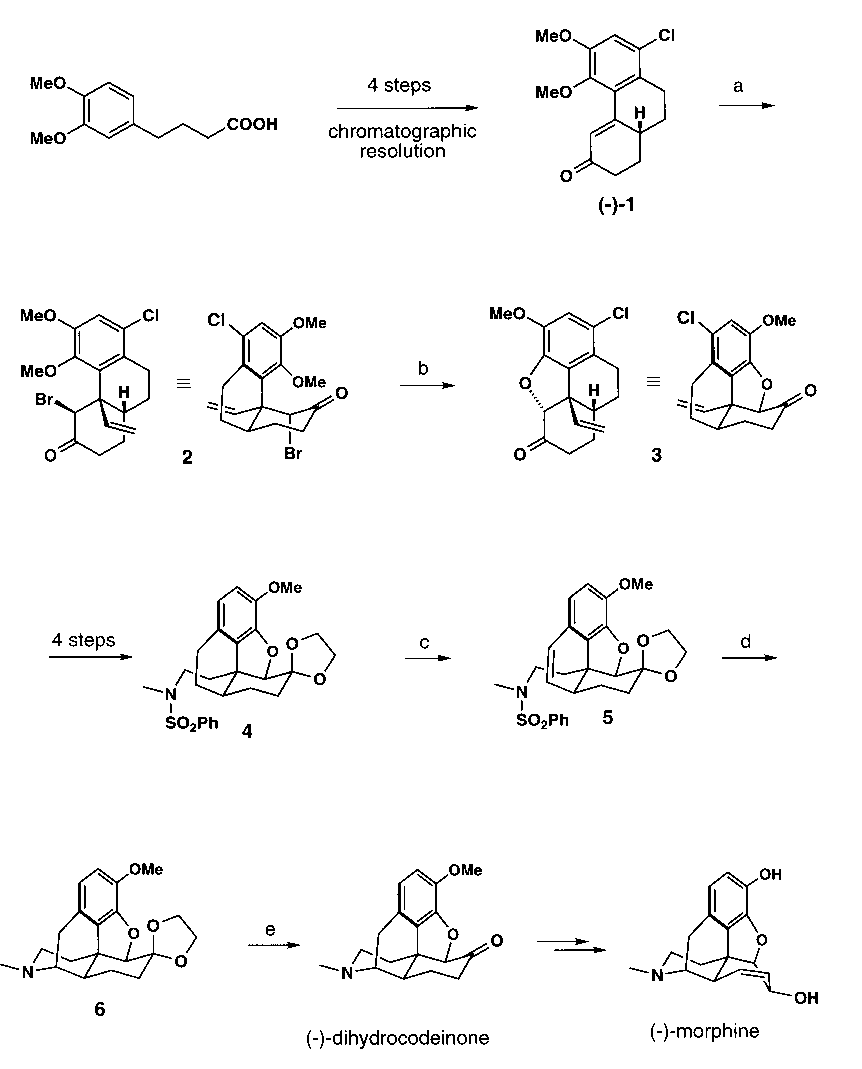
Scheme 1. a) (H2C=CH)2CuMgCl, THF, - 78 °C to 0 °C; ii) TMSCl, Et3N, 0 °C to 25 °C; iii) NBS, THF (63-80%). b) DMF, 140 °C (99%). c) NBS, (PhCOO)2, CCl4, rflx (68 - 81%). d) Li, NH3, THF, t-BuOH (79 %). e) 3 N HCl, 90 °C (95 %).
3. Synthesis via Eschenmoser-Claisen Rearrangement3
In addition to the cuprate chemistry we also used sigmatropic rearrangements
to establish the benzylic quaternary stereocenter (Scheme 2). Diastereoselective
reduction of 1 furnished the allylic alcohol 7 which underwent
Eschenmoser-Claisen rearrangement to afford amide 8. Modification
of the C2-side chain of 8, followed by diastereoselective
epoxidation, then gave sulfonamide 9. Treatment of
9
with trifluoroacetic acid (TFA) in
dry THF effected the desired ring closure with concomitant demethylation
and provided the secondary alcohol 10. Finally, 10 was converted
into the secomorphinane 4, thereby intercepting our previous synthetic
route.
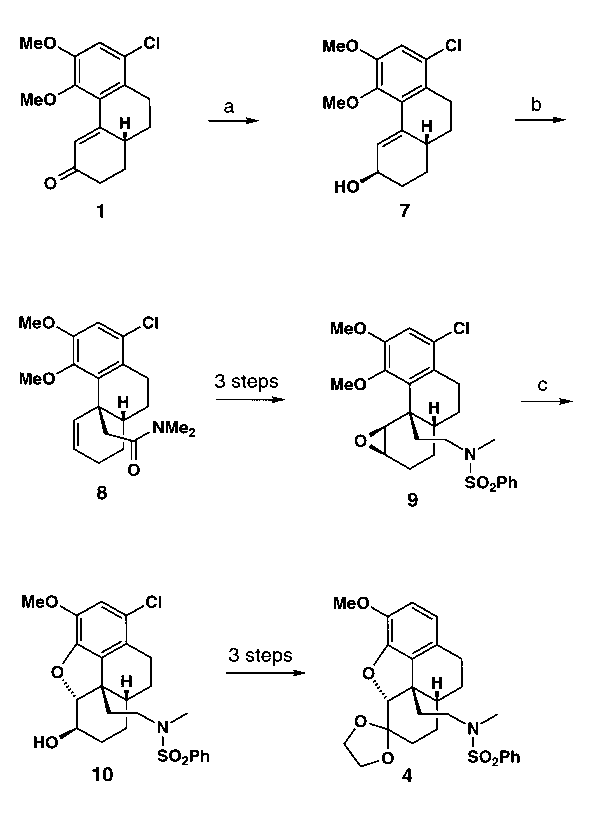
Scheme 2. a)
DIBAH, THF, -78 °C (80%). b) N,N-Dimethyl-acetamide dimethyl
acetal, PhMe, reflux (64%). c) TFA, THF, r.t. (83%).
4. Synthesis of the Hasubanane Skeleton.
Our organocuprate strategy was also applied to the synthesis of hasubanane alkaloids which differ from morphine alkaloids mainly in their pyrrolidine ring (Scheme 3). The conjugate addition of a vinyl cuprate to phenanthrenone 1 - shown here as its (+)-enantiomer - afforded the ketone 11 which was converted to the azidoalkene 13 via compound 12. On heating, 13 underwent clean intramolecular 1,3-dipolar cycloaddition to furnish triazolidine 14. The X-ray crystal structure of 14 in the form of its ethylene ketal is depicted in Figure 3. Treatment of 14 with base resulted in elimination of nitrogen and provided the amino-enone 15 which contains the complete hasubanane skeleton. The refinement of this strategy and the completion of the synthesis of (-)-hasubanonine are currently being pursued in our laboratories.
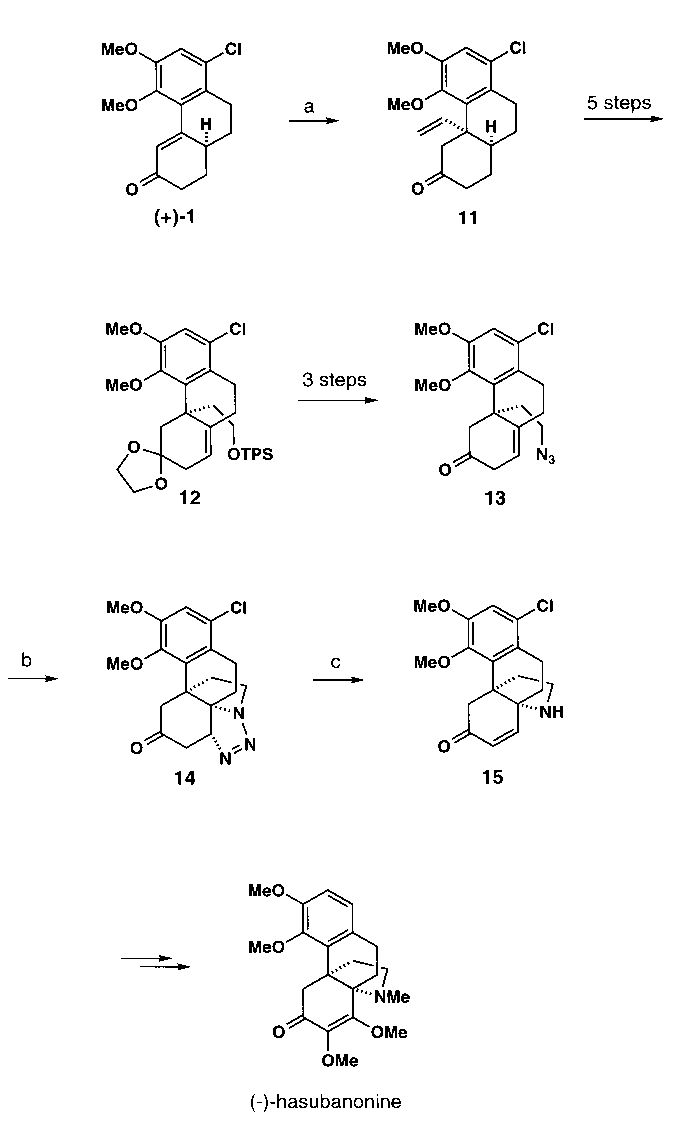
Scheme 3. a)
(H2C=CH)2CuMgCl, THF (91%). b) PhH,
D
(82%). (c) Py,
D
(75%).
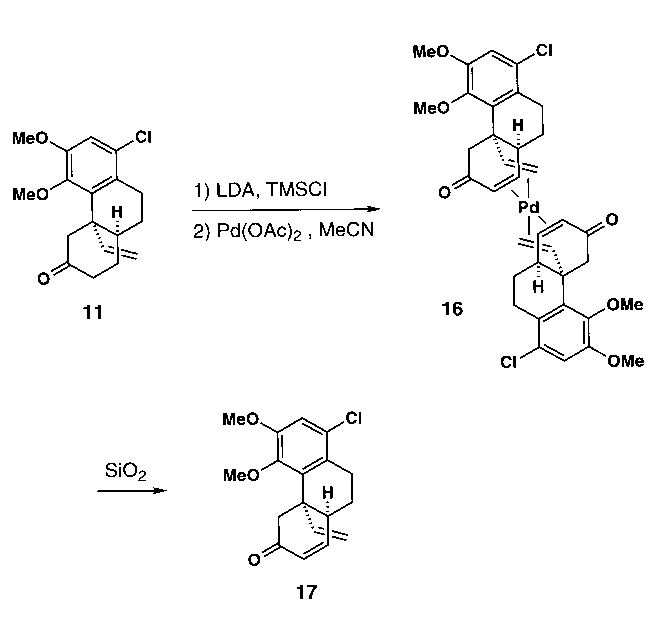
5. A Vinyl Residue Takes Over !
The Saegusa-oxidation of ketone 11 initially yielded
the novel palladium(0)-enone complex 16. Upon silica gel-chromatography,
the expected enone 17 was liberated. The structure of 16 in
the crystal is shown in Figure 4.
Scheme 5
6. Acknowledgment
We thank K. Högenauer for preparing the HTML version of this poster.
7. References
For reviews of the total synthesis of morphine alkaloids, see: a) Hudlicky, T.; Butora, G.; Fearnley, S.P.; Gum, A.G.; Stabile, M.R. in Studies in Natural Products Chemistry (Ed.: Atta-ur-Rahman); Vol. 18, p. 43; Elsevier: Amsterdam 1996. b) Maier, M. in Organic Synthesis Highlights II (Ed.: Waldmann, H.); p. 357; VCH: Weinheim 1995.
Mulzer, J.; Dürner, G.; Trauner, D. Angew. Chemie Int. Ed. Engl. 1996, 35, 2830 [Angew. Chemie 1996, 108, 3046].
Mulzer, J.; Bats, J.W.; List, B.; Opatz, T.; Trauner, D. Synlett 1997, 441.
Comments
During 1-30 September 1997, all comments on this poster should be sent by e-mail to ecsoc@listserv.arizona.edu with A0048 as the message subject of your e-mail. After the conference, please send all the comments and reprints requests to the author(s).