Rev Drone
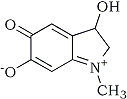
Adrenochrome
Adrenochrome: 3-Hydroxy-1-methyl-5,6-indoline-dione
C9H9NO3 (mw 179.17) CAS No: [54-06-8]
Anybody who's read "Fear and Loathing in Las Vegas" with their eyes open has wondered about that andrenochrome scene. What is that stuff? Where do I get that stuff? etc.
Well, adrenochrome is made by the oxidation of epinephrine (adrenalin). Now I can see where the hydrogens were abstracted from, and I can generally speculate about the mechnism, but does anyone have the definative word on electron-pushing for this little chemical anomaly?
Also, does anyone have experience with this stuff? Johnny Depp sure made it look kewl, and being the young, impressionable lad that I am, I'd sure like to know if it is "all that" or not.
Beagle
I have no experience with this compound, but of course I am very interested in it. Here are the original refs, in case anyone is willing to follow up on it. The original paper, in which Hoffer obtained "profound psychomimetic symptoms" from as little as 0.5 mg SC or IV: J. Mental Sci. 100, 29 (1954)
Later papers where negative results or possible slight effects were obtained by dosages up to 25 mg:
- Am. J. Psychiat. 111, 603 (1955)
- Am. J. Psychiat. 115, 162 (1958)
- Am. J. Psychiat. 116, 454 (1959)
- Lancet II, 308 (1958)
I believe that the ultimate authority of Shulgin refered to adrenochrome as "a fascinating red herring" in Tihkal. And I really hate to argue with Him. I believe that the original, positive reports were based on experiments with one mentally unstable woman. This was covered in Hoffer and Osmonds 'The Hallucinogens', I believe.
Other reviews are:
- Hormones, Brain Function and Behavior, p. 181 (1957)
- Psychotropic Drugs p. 10 (1957)
Opinions that adrenochrome is not active can be found in Lancet I, p. 1287 (1960). In Nature 191, 245 (1961) it was pointed out that adrenochrome may have significance as a hallucinogen, since it is an in vitro glutamic acid decarboxylase and cholinesterase inhibitor. It has also been described as having a wide range of other physiological activites, as reviewed in Fortschr. Chem. Org. Naturstoffe. 14, 217 (1957) and Pharmacol. Rev. 1, 1, (1949).
Of course, most of this work was done under conditions that would be considered primitive today, and there is not absolute certainty that all research groups were working with the same compound. Originally, adrenochrome was prepared by oxidation of adrenalin with catechol oxidase or silver oxide. The chemistry of its preparation is reviewed in Chem. Rev. 59, 181 (1959)
Additionally, adrenochrome semicarbazone has been claimed to have hallucinogenic properties similar to the parent substance: Acta Chem. Scand. 12, 1231 (1958). It certainly would be interesting to apply modern techniques to this mystery and settle it once and for all. Anyone looking for a research project?
Beagle
I would be glad to hear of any other epinephrine oxidations that you could offer. Also, for another fun fictionalized account of adrenochrome in the vein of H. S. Thompson, see Blood of a Wig, from the book Red Dirt Marijuana and Other Stories, by Terry Southern.
I just checked Medline, and low and behold, there has been quite a bit of work done in recent years on the physiological significance of adrenochrome.
One article that I saw on the mechanism of this transformation was Biol. Signals, 5(5), 275-82 (1996), where it was suggested that the transformation is an enzyme mediated one, and is dependant on H2O2 and O2-. NADH also apparently stimulates the production of adrenochrome, indicating involvement of NADH dehydrogenase. I had no idea that this was still a current topic of research, but there were over 100 cites, just going back to 1986.
Further searching of Medline has yielded the following papers relating to adrenochrome and mental function that may prove interesting. I don't have easy access to any of them. If anyone gets them and finds anything interesting, please post.
Molecular interactions in the phenomenology of the onset of mental illness
Chem-Biol-Interact. 7(1), 1-9 (1973)
[Chemical interaction between serotonin and adrenochrome.
Contribution to the "short circuit" theory of the genesis of mental disease]
Riv-Neurol. 41(3), 182-7 (1971)
Short circuit theory on the onset of mental illness
Clin-Chim-Acta. 30(1), 5-11 (1970)
Mobius
Adrenochrome Semicarbazone
Adam Gottlieb
'Legal Highs', 20th Century Alchemist (1973)
Usage: 100mg is thoroughly dissolved in just enough alcohol, melted fat (butter), or vegetable oil and ingested.
Because of its poor solubility in water these must be used to aid absorption.
Effects: Physical stimulation, feeling of well-being, slight reduction of thought processes.
Contraindications: None noted. Act as a systemic hemostatic preventing capilary bleeding during injury.
Adrenochrome causes chemicaly induced schizophrenia. It's semicarbazone does not...
Agnememnon
[Review of adrenochrome as a psychotomimetic agent]
R.A. Heacock
Chim. Ther. 6, 300 (1971)
[Preparation of adrenochrome using Ag2O as an oxidising agent]
MacCarthy
Chim. Ind. Paris 55, 435 (1946)
Teonanacatl
I was curious if any of you have looked into the chemistry of adrenochrome... I just happened across a paper by Heacock, and it really interested me, but I haven't been able to find any other literature on it...I realize that it isn't fully an indole, but it is pretty close... The paper I've been reading mostly goes through it's chemistry (quite detailed...) and I was looking for some reviews of physiological action to round out my knowledge.
Here's the ref one I was looking at:
The Chemistry of Adrenochrome and Related Compounds
R. A. Heacock
Chem. Rev. 59, 181 (1959)
Synthesis and Chemical Properties of Adrenochrome
XIII. Properties of adrenochrome.
Biochemical Journal 31(1), 609 (1937)
Adrenochrome is a very unstable substance; it is completely destroyed in 4 min by 1% HCl and in 40 sec. by 1% NH4OH. At pH 7.3 and 37°C the red color disappears in 35 min, and even under the optimium conditions for keeping the solution, at pH 4.0 and 0°C, marked decompostion and melanin formation could be observed in 4-5 hours. In the dry crystalline condition adrenochrome could be kept for several weeks, but on standing in a vacuum desiccator it decomposed to give a brown amorphous product. The crystalline substance appears to be a hydrate. The crystals decompose on heating at abot 115-120°C.
Adrenochrome is very soluble in water and crystallizes from water only on evaporation practically to dryness. It is also readily soluble in methyl alcohol. It is removed quantitatively from the aqueous solution by adsorption on charcoal, and can be eluted again from the charcoal with methyl alcohol.
Adrenochrome by AgO Oxidation of Adrenaline
J. Chem. Soc. 1276-1279 (1950)
(-)-Adrenaline (2 g) was suspended in water (70 mL) and dilute hydrochloric acid was added dropwise with shaking, until a clear solution was
obtained. Silver oxide (8 g) was added, the mixture shaken vigorously for 5 minutes and then filtered. The aqueous solution of adrenochrome thus obtained
was stable for about 12 hr at 0°C.
Chemistry & Mysticism
"...Then came the discovery that adrenochrome, which is a product of the decomposition of adrenalin, can produce many of the symptoms observed in mescalin intoxication. But adrenochrome probably occurs spontaneously in the human body. In other words, each one of us may be capable of manufacturing a chemical, minute doses of which are known to cause Profound changes in consciousness..."
-- Aldous Huxley
The Doors of Perception
Chemistry and mysticism [...] The question of religious chemistry has been underscored recently by the wide attention given to the theories, already mentioned, of Dr. Abram Hoffer and Humphry Osmond. Their adrenochrome-adrenolutin hypothesis suggests that schizophrenia may be caused at least in part by defective adrenal metabolism. Very briefly, the adrenal gland secretes the hormone adrenaline, which helps coordinate biological mechanisms in emergency situations, for example, a fist fight or a threatened traffic accident. Heart rate is increased, the blood is sugared up and pumped to the necessary muscles. Adrenaline also may affect the emotions, contributing to anxiety and depression. In the body it turns into a toxic hormone called adrenochrome, which in turn can be converted into either of two other compounds: dihydroxyindole or adrenolutin. It is possible that dihydroxyindole balances off adrenaline to reduce tension and irritability; in schizophrenics, however, adrenochrome is converted primarily into adrenolutin, which also is toxic, and the combination of adrenochrome-adrenolutin results in a poisonous disruption of the brain's chemical processes. That is the theory. And the prescribed antidotes are nicotinic acid (niacin) or nicotinamide (Vitamin B-3). Discussing one of the villains in the piece, the scientists write: "There are few who doubt that adrenochrome is active in animals or in man, and it is now included among the family of compounds known as hallucinogens - compounds like mescaline and LSD-25 capable of producing psychological changes in man."
Excerpt from "The Private Sea" by William Braden
rev drone
The following references were collected from Beilstein Crossfire.
Reaction Type: Preparation
Reactant: 4-((R)-1-hydroxy-2-methylamino-ethyl)-benzene-1,2-diol Product: (R)-3-hydroxy-1-methyl-indoline-5,6-dione Reagent: silver oxide, methanol Ellis; J.Pharmacol.Exp.Ther.; 79; 1943; 364,368; Veer; Recl.Trav.Chim.Pays-Bas; 61; 1942; 638,643;
Reagent: silver oxide, ethanol Ellis; J.Pharmacol.Exp.Ther.; 79; 1943; 364,368; Veer; Recl.Trav.Chim.Pays-Bas; 61; 1942; 638,643;
Reagent: silver oxide, methanol, formic acid Heacock et al.; Can.J.Chem.; 36; 1958; 853,855; Sobotka; Austin; J.Amer.Chem.Soc.; 73; 1951; 3077; Harley-Mason; J.Chem.Soc.; 1950; 1276,1279; Ingle et al.; J.Am.Pharm.Assoc.; 37; 1948; 375; MacCarthy; Chim.Ind.(Paris); 55; 1946; 435;
Reagent: oxygen Other conditions: in Gegenwart von Katalysatoren Green et al.; J.Biol.Chem.; 220; 1956; 237; Chaix et al.; Biochim.Biophys.Acta; 5; 1950; 472,473; Drevon; Stupfel; C.r.Soc.Biol.; 143; 1949; 271; Blaschko; Schlossmann; J.Physiol.London; 98; 1940; 130-140; Green; Richter; Biochem.J.; 31; 1937; 598,609;
Reagent: Fe2+, hydrogen peroxide Harley-Mason; J.Chem.Soc.; 1950; 1276,1279; Mazur et al.; J.Biol.Chem.; 220; 1956; 227;
Reagent: Fe2+, sodium peroxodisulfate Harley-Mason; J.Chem.Soc.; 1950; 1276,1279; Mazur et al.; J.Biol.Chem.; 220; 1956; 227;
Reagent: water, iodic acid Correia; Alves; Anais Fac.Farm.Porto; 14; 1954; 101,109; Macciotta; Gazz.Chim.Ital.; 81; 1951; 485,488;
Reaction Type: Chemical Behaviour
Reactant: 4-(1-hydroxy-2-methylamino-ethyl)-benzene-1,2-diol Product: 3-hydroxy-1-methyl-2,3-dihydro-indole-5,6-dione Reagent: VOCl2, O2 Solvent: H2O Temp: 25°C Other conditions: dependence on pH, conc of VOCl2 and adrenaline; ionic strength: 0.100M (KNO3) Subject studied: Rate constant Jameson, Reginald F.; Kiss, Tamas; J.Chem.Soc.Dalton Trans.; 1986; 1833-1838;
Other conditions: disproportionation during electrooxidation at carbon electrodes at phys. pH Subject studied: Rate constant, Mechanism Ciolkowski, E.L.; Maness, K.M.; Cahill, P.S.; Wightman, R. M.; Evans, D.H.; Anal.Chem.; 66; 21; 1994; 3611-3617
Reactant: 4-(1-hydroxy-2-methylamino-ethyl)-benzene-1,2-diol Product: 3-hydroxy-1-methyl-2,3-dihydro-indole-5,6-dione, 1-methyl-indole-3,5,6-triol Solvent: D2O, H2O Time: 350 min Temp: 24.9°C Other conditions: Irradiation other sensitizers: methylene blue, rose bengal, fluoresceine, superoxide dismutase; other inhibitors: 1,3-diphenylisobenzofuran, 1,4-diazobicyclo<2,2,2>octane, KN3, NaN3 Subject studied: Rate constant, Mechanism Kruk, I.; Z.Phys.Chem.(Leipzig); 268; 3; 1987; 607-613
Reactant: 4-(1-hydroxy-2-methylamino-ethyl)-benzene-1,2-diol Product: 3-hydroxy-1-methyl-2,3-dihydro-indole-5,6-dione, C9H11NO3 Other conditions: Irradiation, laser induced electrooxidation at pH5; half life of O-quinone Subject studied: Product distribution Skully, Joan P.; McCreery, Richard L.; Anal.Chem.; 52; 12; 1980; 1885-1889;
Reactant: C9H11NO3 Product: 3-hydroxy-1-methyl-2,3-dihydro-indole-5,6-dione Subject studied: Rate constant, half life Skully, Joan P.; McCreery, Richard L.; Anal.Chem.; 52; 12; 1980; 1885-1889;
Reactant: (±)-1-<3,4-dihydroxy-phenyl>-2-methylamino-ethanol Product: C9H9NO3 Reagent: K3Fe(CN)6 Solvent: acetic acid, H2O Time: 5 min Temp: 5°C Kato, Eishin; Oya, Masayuki; Uda, Kozo; Iso, Tadashi; Fujita, Tadashi; Iwao, Jun-Ichi; Chem.Pharm.Bull.; 29; 7; 1981; 1935-1941;
Reactant: C9H11NO3 Product: (R)-3-hydroxy-1-methyl-indoline-5,6-dione Subject studied: Rate constant Reagent: Fe(NO3)3, KNO3 Solvent: H2O Temp: 25°C Linert, Wolfgang; Herlinger, Erwin; Jameson, Reginald F.; J.Chem.Soc.Perkin Trans.2; 12; 1993; 2435-2439;