This synthesis is a mercuration reaction, where a ionic mercuric salt adds across the double bond of allylbenzene together with a nucleophile (in this case a phosphoramidate), which is followed by reduction by sodium borohydride, whereupon the organic mercury compound is converted to a hydrocarbon and elemental mercury. The resulting diethylphosphoramide of amphetamine is then hydrolyzed to amphetamine with anhydrous hydrogen chloride in benzene.
The reaction should be general in scope and work for all allylbenzenes. The major drawback of this procedure is that it uses a stoichiometric amount of mercury nitrate, which is a pretty large amount considering its molecular weight compared to the alkene. Mercury nitrate is very toxic, and the intermediate organomercury compound is even more toxic, as it more easily can be taken up in the body than inorganic mercury salts. After the NaBH4 reduction, the mercury will be in the elemental form, and must be disposed of properly (either by recycling it yourself by making new mercury nitrate from it, or leaving it to a recycling station where they will take care of it and prevent its release into the environment).
It is of utmost importance that the product amphetamine is thoroughly purified to remove any possible traces of organomercurials if the product amphetamine is intended for human consumption. Distillation of the freebase amine followed by recrystallization of the hydrochloride salt is an absolute must for health and safety reasons, even though it's not indicated in the procedure below. The synthetic description is a general reaction scheme for any alkene, and that the original authors didn't intend to consume their products is very likely a safe assumption.
Experimental
General Procedure:
Diethyl N-Alkylphosphoramidates
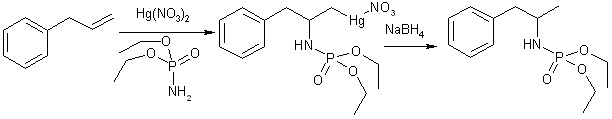
A mixture of diethyl phosphoramidate (9.2 g, 60 mmol), dry mercury(II)nitrate (6.67 g, 20 mmol), the alkene (20 mmol), and 1,1-dichloroethane (60 ml) is refluxed gently with stirring for 4 h. The resultant yellow-orange solution is cooled to 0°C and then aqueous 10% sodium hydroxide (60 ml), and a solution of sodium borohydride (0.8 g, 20 mmol) in aqueous 10% sodium hydroxide (20 ml) are added. Stirring is continued for 1 h at room temperature. The precipitated mercury is filtered off and washed with dichloromethane (30 ml). The organic layer is thoroughly washed with water (3x20 ml), dried with magnesium sulfate, and evaporated. The residual crude phosphoramidates mostly are analytically pure when heated at 40-50°C/0.2 torr for 1 h to remove traces of volatile impurities.
General Procedure:
Hydrolysis of Diethyl N-Alkylphosphoramidates
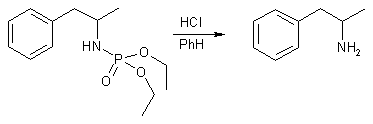
10 mmol of the crude phosphoramidate is left to stand overnight in 30 ml of benzene saturated with dry HCl gas. The benzene and excess HCl is evaporated and the residue is added a solution of 40g of NaOH in 50 ml of water and the liberated amine is steam-distilled. The distillate is made alkaline (pH 12-14), saturated with solid sodium chloride, and extracted with ether (3 x 50 ml). The extract is dried with magnesium sulfate, saturated with gaseous hydrogen chloride. Evaporation to dryness followed by addition of hexane (10 ml) affords crude amine hydrochlorides [70% yield for amphetamine] which can be recrystallised if necessary.
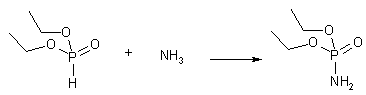
Chemicals
Diethyl phosphoramidate
Obtained by leading dry NH3 gas through diethyl phosphite in carbon tetrachloride.2
Mercury(II) Nitrate
Commercial mercury(II)nitrate hemihydrate [Hg(NO3)2·½H2O], which is strongly hygroscopic and containing varying amounts of water, is ground in a mortar and dried in a vacuum dessiccator over phosphorus pentoxide (P2O5) for several days, and then stored over P2O5 to avoid water being re-absorbed.