4-Allyl-Pyrocatechol through Base-catalyzed Claisen Rearrangement1
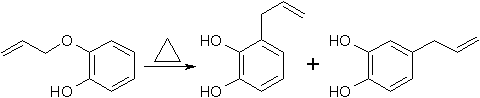
The thermal rearrangement of catechol mono-allyl ether is reported2,3,4 to give at 160-200°C a mixture of products, 3-allyl-catechol and 4-allylcatechol, in the ratio 5:4, apparently resulting from competing ortho- and para-Claisen rearrangements5,6. Our investigation of this reaction shows that the ratio of the two products and the rate of the rearrangement are sensitive to the pH at which the reaction is carried out. Thus in ethanolic HCl (2 drops HCl in 25 ml EtOH) at 180°C the ratio of 3-allyl- to 4-allylcatechol is 2:1 and in ethanol containing 1 equiv. of NaOEt at 78°C the reaction proceeds at approximately the same rate and the ratio 3-allyl- to 4-allylcatechol is 1:4. These preliminary observations suggested that the rearrangement of catechol mono-allyl ether is base catalysed and that the rearrangement of the anion of catechol mono-allyl ether takes a different course from that of the un-ionised phenol.
Experimental1
Equimolar amounts of catechol mono-allyl ether and sodium ethoxide dissolved in anhydrous ethanol as solvent (2-3 times the weight of the ether). Reflux for a few hours, evaporate the solvent and take up the residue in 2M NaOH. Wash the basic solution with DCM to remove by-products, then acidify to pH <3 and extract thrice with DCM. Evaporate the solvent and fractionately distill the residue to separate the allyl-catechols.
Allylpyrocatechols3
When 100g Pyrocatechol mono-Allyl Ether was heated 35-40 min at 160-170°C an exothermal reaction with a temperature jump to 270°C took place, producing a liquid which on fractional distillation, gave 35-38% 3,4-dihydroxy-1-allylbenzene (4-allylcatechol) bp 123-5°C/1-2mmHg, mp 48°C, and 47.5% 2,3-dihydroxy-1-allylbenzene (3-allylcatechol) bp 95°C/5mmHg, mp 24°C.
Physical properties of 4-allylcatechol
Alternate names: 3,4-dihydroxyallylbenzene, 4-allylpyrocatechol.
Melting point: 46-48.5°C
Boiling point: 123-124°C/1mmHg, 141-144°C/7mmHg, 147-149°C/10mmHg, 155-157°C/13mmHg, 156-158°C/16mmHg.
Solubility (H2O): 25 g/l at 26°C.
Recrystallization solvents: Benzene, petroleum ether, diethyl ether.
Pyrocatechol mono-Allyl Ether (2-allyloxyphenol)
Using Allyl Bromide in Acetone7
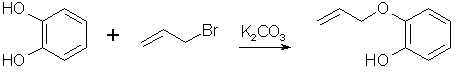
110g (1 mole) catechol and 138g potassium carbonate (1 mole) was mixed in 200ml of acetone, with some evolution of heat. The flask containing this mixture was then fitted with a stirrer and attached to a reflux condenser. 121g (1 mole) of allylbromide was added slowly and with constant stirring. When about three-fourths of it had been added, external cooling of the flask was necessary to prevent too vigorous boiling. The remaining allylbromide was added with less evolution of heat. Refluxing on a water bath with continual stirring was continued for four hours. After cooling, the solid material was filtered off and washed with 50 mL of acetone. The washing were added to the first filtrate and the acetone was removed by distillation. The oil was then fractionally distilled under vacuum and the portion coming over at 108-113°C/18 mmHg was collected in a 70% yield.
The allyl ether must be vacuum distilled under a good vacuum (at least 25 mmHg), or else it will rearrange.
Using Allyl Chloride & Sodium Iodide in Acetone3
100g Pyrocatechol (0.9 mol), 100g allyl chloride (1.3 mol), 15g NaI (0.1 mol) and 106g Na2CO3 (0.77 mol) was refluxed in anhydrous acetone to give a 75% yield of pyrocatechol monoallyl ether, bp 81°C/1.5 mmHg.
Acetone is the preferred solvent for the reaction. In ethanol the reaction only gave approximately 45%, as did it in propanol (without the NaI), and in propanol with the NaI added the yield was 68%.
Cognate Syntheses:
Allylation of guaiacol (2-methoxyphenol) with allyl bromide, followed by Claisen rearrangement of the formed 2-allyloxyanisole gives 2-hydroxy-3-methoxyallylbenzene (o-Eugenol) in good yield8. This can in turn be used as a precursor for asarone and other interesting compounds.