I. Introduction
"To conquer disease and achieve a surcease from pain, man has ransacked the entire earth for drugs. And what he could not find in nature the chemist created in his laboratory. But many of these useful drugs are like the finger of God — they can heal and they can smite."1 The misuse of drugs has played a role in society at least as far back as Hippocrates and was reflected in the vocabulary of the times: pharmakon — a drug, medicine, or poison; pharmacopeus — a purveyor of toxic substances; pharmakopoloi — traveling quack doctor; pharmakoi — condemned criminal.2 The problems of drug use and misuse are still of concern, and health hazards posed by the availability of designer drugs is a major focus today.3
"Designer drugs are analogs, or chemical cousins, of controlled substances that are designed to produce effects similar to the controlled substances they mimick. By slightly altering the chemical formula of a controlled substance....a new drug is created which will produce the high or euphoria the user wants." This definition was given during Congressional hearings4 on designer drugs in 1985 where the illicit drug trade was estimated to be a $110 billion per year industry in the U.S., which is about three times the total sales of the U.S. pharmaceutical industry.5 The illicit importation was an estimated 10 tons of heroin, 85 tons of cocaine, and 15,000 tons of marijuana. Further, "The production of illicit drugs abroad continues unabated, and in our own country illicit marijuana has become a major cash crop."4 The designer drug portion of the illicit market is estimated at $1 billion and primarily in opiate-type and phenylisopropylamine-type street drugs at the current time.3
Why is there drug abuse? Why do people consume designer drugs or other illicit drugs? The answer commonly given: because it feels good. The drugs that are so used are psy choactive drugs, ones that produce effects on thought, feeling, mood, self-perception, and give a sense of "high" or intoxication. Self-administration of drugs for nonmedical, indeed, recreational purposes can be a hazard as it may lead to compulsive or addictive use. Drugs from the illicit marketplace have the additional hazard of containing impurities or untested chemicals that can be toxic and life threatening. Efforts to understand why opiates and hallucinogens are self-administered yields the following descriptions. Intravenous use of an opiate produces warm flushing of the skin and sensations in the lower abdomen described by addicts as similar in intensity and quality to sexual orgasm. Consumption of a hallucinogen drug, or other central nervous system sympathomimetic drugs, such as cocaine, produces a heightened awareness of sensory input, often with an enhanced sense of clarity, and the user receives vivid and unusual sensory experiences, attention is focused inward with a sense of union with the cosmos or mankind. The phenomenon of drug abuse has become a sophis ticated and relatively new area of study of the human being from perspectives of physiology and psychology, where concern is given to such issues as tolerance and cross tolerance, dependence and addiction, and induced behavioral reinforcement that will certainly lead to a better understanding of the human organism.6
The design of new drugs often utilizes basic principles of the chemistry laboratory whereby the structure of a drug molecule is slightly altered in order to alter the pharmacological activity. This principle of structure-activity relationships, known as SAR, has been applied to many medically approved drugs that are in the marketplace. For the opiates, SAR studies have been pursued in the search for a nonaddicting analgesic for the treatment of pain. Slight structural changes have produced new drugs with altered receptor-binding properties and altered potency or activity. Basic research on the opiates during the last decade has lead to important scientific discoveries of multiple opiate receptors, the endogenous opioids, and renewed interest in the structure and function of polypeptides.7 The hallucinogen family of drugs are not as well understood as the opiates. A 1978 conference on quantitative SAR studies of the opiates and hallucinogens found detailed discussions on opiate receptor binding and sophisticated arguments as to the existence of multiple opioid receptors with different pharmacologic properties, while discussions on the hallucinogens were in a relatively early stage of development.8 There are substantial benefits to be gained from a basic understanding of how drugs of this family, many of which can be classified as sympathomimetic drugs together with cocaine, interact and/or interfere with the chemical messengers or neurotransmitters of the autonomic nervous system.
Clandestine production of drugs, so called street drugs, is intended to avoid federal regulation and control. The result is availability of unknown substances of unknown purity that may have the potential to cause serious toxicity with potentially dangerous health consequences for the naive drug user. A review of illicit drugs and analogues produced by the clandestine pharmaceutical industry concludes that the quality of personnel involved in drug synthesis ranges from cookbook amateurs to highly skilled chemists. And quite surprisingly, "most of these new analogs have been previously reported in the literature with animal data that suggest they would be reasonably active and have similar pharmacological effects to the lead compound in the series".9
II. Regulatory Efforts at Drug Control
For various reasons, governments have been attempting to control the sale and use of drugs for many years at both the national and international levels. Efforts to provide a legal framework in order to guide and coordinate the nations in the control of psychoactive drugs began in 1909 with an international commission to control the opium poppy plant. In 1961 control efforts extended to the coca bush and the marijuana plant, and finally in 1971 to synthetic substances. Within the world community, the 1961 Single Convention on Narcotic Drugs, together with the amending Protocol of 1972, and the 1971 Convention of Psychotropic Substances are the primary pieces of international legislation that set up the mechanisms for coordinated control of dependence-producing and mind-altering drugs. A list of these treaties is present in Table 1.10
Table I
International Drug Control Treaties
- 1909 - The Shanghai International Opium Commission
- The first international body concerned with narcotic drugs
- 1912 - The Hague International Opium Convention
- Established intemational cooperation in the control of narcotic drugs
- 1925 - International Opium Convention
- Established a system of import and export requirements for trade in narcotic drugs
- 1931 - Convention Limiting the Manufacture and Distribution of Narcotic Drugs
- Limited manufacturing to meet medical and scientific needs
- 1936 - Convention on Suppression of Illicit Traffic in Dangerous Drugs
- Set up punishment for illicit drug traffickers
- 1946 - Protocol
- Transferred to the United Nations functions previously held by the League of Nations
- 1948 - Protocol
- Brought under control selected synthetic substances that were dependence producing
- 1953 - Opium Protocol
- Further restricted the amounts of opium for trading and those nations which were permitted do so
- 1961 - Single Convention on Narcotic Drugs
- Modernized systems of multilateral treaties on control
- Formed the International Narcotics Control Board
- Extended controls to include the coca bush and marijuana plant
- Prohibited opium smoking and eating, coca leaf chewing, and cannabis smoking
- Provided for medical treatment and rehabilitation of abusers
- 1971 - Convention on Psychotropic Substances
- Extended the Single Convention of 1961 to control central nervous system stimulants, sedative-hypnotics, and hallucinogens
- Introduced provisions for prevention and treatment of drug abuse
- 1972 - Protocol Amending the Single Convention on Narcotic Drugs
- Intensified efforts to prevent illicit production, traffic in, and use of narcotic drugs; emphasized prevention through information and education; emphasized need for measures for treatment, rehabilitation, and social reintegration of abusers
- Strengthened role of International Narcotics Control Board in reducing demand, supply production, manufacture, traffic, and use
- 1981 - International Drug Abuse Control Strategy
- Adopted Global Plan for strategy and 5-year program for action to deal with the growing drug abuse problem.
The U.S. government has established a series of legislative controls on drug use and production in order to safeguard the public. These efforts are extended over both control of drug misuse or abuse and over the production of new drugs for medical therapeutic use to improve the quality of life. The various legislative acts are listed in Table 2. Throughout the legislative history there was an obvious concern for pure drugs that are safe and effective. The creation of the Food and Drug Administration (FDA) has provided for a more rational manner of discovery and development of new chemical entities for medical use. It is instructive to briefly review the FDA procedures in order to be aware of the extensive efforts that are required in order to assure, as much as is possible, that a drug is safe before it is permitted for general use by the public.
Table 2
National Drug Control Legislation
- 1906 - Pure Food and Drug Act
- Prohibited mislabeling and adulteration of drugs
- 1909 - Opium Exclusion Act
- Prohibited the importation of opium
- 1912 - Amendment to the Pure Food and Drug Act
- Prohibited false and midleading advertising
- 1914 - Harrison Narcotics Act
- Established regulations for the use of opium,
opiates, and cocaine
- 1937 - Amendment to the Harrison Narcotics Act
- Established regulations for the use of marijuana
- 1938 - Food, Drug, and Cosmetic Act
- Required that new drugs be safe as well as pure
- 1952 - Durham-Humphrey Act
- Gave power to the FDA to determine which
products could be sold without prescription
- 1962 - Kefauver-Harris Amendment to the
Food, Drug, and Cosmetic Act
- Gave power to the FDA to require proof of
efficacy for new drugs
- Established guidelines for reporting information
on adverse reactions, clinical testing, and
advertising of new drugs
- 1970 - Controlled Substances Act
- Established DEA authority to classify drugs
according to abuse potential and to control
their distribution
- 1984 - National Narcotics Act
- Provided authority for quickly adding new
controlled substance analogues to Schedule
of Controlled Substances
The U.S. imposed special restrictions on those drugs with potential for abuse under the Comprehensive Drug Abuse Prevention and Control Act of 1970, which is commonly referred to as the Controlled Substances Act. Under this legislation the Drug Enforcement Agency (DEA) has designated and classified all drugs with abuse potential. There are five schedules of controlled substances known as Schedules I, II, III, IV, V, and within each schedule are lists of specific drugs including narcotics, stimulants, hallucinogens, and depressants. The drugs in Schedule I are those that have a high abuse potential and include heroin, marijuana, and the hallucinogens. These are drugs that have no currently accepted medical use, thus a physician will have no concern with Schedule I unless he is involved in conducting research. For Schedule II drugs, which also have a high abuse potential, the physician cannot give telephone prescriptions nor refills. Schedule III drugs, which are classified as having a lower abuse potential than drugs in I or II, are limited such that medical prescriptions must be rewritten after 6 months or 5 refills. The drugs listed in Schedule IV, classified as moderate potential for abuse, have the same restrictions as those in III except that the penalty for illegal possession is less severe. Schedule V drugs have a low abuse potential and may be dispensed without a prescription unless otherwise specified by state law. The list and classification of substances is in accord with the international conventions for psychotropic drugs. The FDA routinely evaluates those new drug candidates that may have a possible potential for producing dependence in order to determine abuse liability.
III. Role of the Food and Drug Administration
The Food and Drug Administration (FDA) became the administrative body to oversee drug evaluation and give official approval to market new drug products within the U.S. In order to begin studies in humans it is first necessary to file an Investigational Exemption for a New Drug (IND) with the FDA. The IND includes detailed information on the composition and source of the drug, manufacturing information, data from animal studies to determine possible acute toxicity, and clinical plans for the studies to be done. Once the IND is granted, clinical studies may be initiated in order to obtain sufficient data to submit a New Drug Application (NDA) to the FDA to gain approval for marketing. Such studies, which are extremely time consuming and costly, are carried in three phases. In Phase 1, clinical trials are done to establish dose-effect relationships in a small number of healthy volunteer subjects. Usually, chronic animal studies are also initiated at this time to establish drug safety and possible toxicities due to long-term use. Phase 2 studies are then carried out with patients, those that have the disease to be treated, in order to establish safety and efficacy in this special population. Phase 3 studies are finally carried out in large numbers of patients in the expected clinical setting to be encountered. In Phase 1 both the investigators and volunteer subjects are aware of the drug treatment being given. Phase 2 studies are usually done with a single-blind protocol where the physicians are aware of the treatments given, but the patients are unaware of what dose is being given or whether they are receiving a placebo (no drug control) or an older known successful drug treatment (a positive control) for comparision purposes. In Phase 3 clinical trials both the physician and patient are unaware of which treatment is being given, a double-blind treatment protocol, in an effort to minimize uncertainty in determining drug efficacy and possible toxic side effects. When these studies are successfully completed and a careful review and evaluation of the data has been carried out, the FDA will grant approval of the NDA and the new chemical entity can be marketed for sale. At this time the new drug enters Phase 4 where postmarketing surveillance is done to obtain further data on the safety and efficacy of the drug once it is in general use. With an extremely large number of subjects it is then hoped to detect as soon as possible any as yet unobserved harmful properties of the new drug.
IV. The Structure-Activity Relationship (SAR)
Chemical strategies to design new drugs have a long and successful history in medical chemotherapeutics. Many useful new drugs or modifications to older drugs have clearly resulted in improved health care for countless persons throughout the world. A good example may be that of Valium® and the benzodiazepine family of drugs. This group of widely used Schedule IV drugs, known as sedative-hypnotic or antianxiety agents in medical treatment, act on the central nervous system.11
| Benzodiazepine |
R1 |
R2 |
R3 |
R7 |
R2' |
| Chlordiazepoxide | - | -NHMe | -H | -Cl | -H |
| Clonazepam | -H | =O | -H | -NO2 | -Cl |
| Clorazepate | -H | =O | -CO2- | -Cl | -H |
| Demoxepam | -H | =O | -H | -Cl | -H |
| Diazepam | -CH3 | =O | -H | -Cl | -H |
| Flurazepam |
-(CH2)2NEt2 | =O | -H | -Cl | -F |
| Halazepam | -CH2CF3 | =O | -H | -Cl | -H |
| Lorazepam | -H | =O | -OH | -Cl | -Cl |
| Nitrazepam | -H | =O | -H | -NO2 | -H |
| Nordazepam | -H | =O | -H | -Cl | -H |
| Oxazepam | -H | =O | -OH | -Cl | -H |
| Prazepam | -(CH2)2C3H6 | =O | -H | -Cl | -H |
| Temazepam | -CH3 | =O | -OH | -Cl | -H |
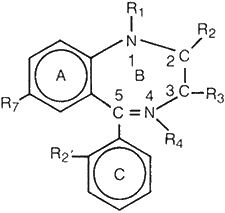
Figure 1.
The chemical names and structures are
given for some benzodiazepine-type
drugs. Note that there is no substituent
at position R4, except for demoxepam
and chlordiazepoxide which are N-oxides.
Selected benzodiazepine drugs with their molecular structures and chemical names are listed in Figure 1 and present a good example of SAR concepts. The diagram drawn at the top of the figure is the chemist's symbolic method of representation for the general family of drugs that belong to the benzodiazepine class of molecules. The general structure consists of three rings denoted by the letters A, B, C, together with a number of locations on the drug molecule where specific atoms or groups of atoms are introduced at the sites labeled R1, R2, R3, R7, R2'. For example Valium® has the chemical name diazepam, and the molecular structure is designated by replacing R1 by the atoms CH3, called a methyl group by the chemist, replacing R2 with =O, a keto oxygen atom, replacing both R3 and R2' with a hydrogen atom H, and replacing R7 with Cl, a chlorine atom.
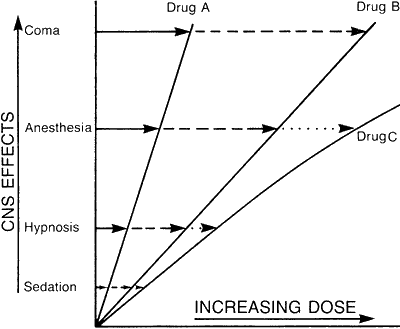
Figure 2.
Schematic dose-effect curves for sedative-hypnotic drugs.
Slight alterations in the substituents at the R sites result in new molecular structures, giving different drugs that have different pharmacological activities; thus, the term structure-activity relationship. In the case of diazepam or Valium®, a change in R, from CH3 to H results in the drug nordiazepam. Then a second change in R3 from H to OH, the hydroxy group, results in the drug oxazepam or Serax®. And continuing further, a change at R2' from H to Cl results in the drug lorazepam or Ativan®. Similarly, changing nordiazepam at R7 from Cl to NO2, the nitro group, gives nitrazepam or Mogadon®. Further, a change at site R2' from H to Cl gives the drug clonazepam or Clonopin®. In medical treatment each drug has a different recommended dosage for safe and effective use. Likewise, each drug has its own dose-effect relationship, as shown schematically in Figure 2.12
It is not a simple task to carry out the types of changes on the structures of molecules as the above reading might imply. Alterations of molecules depend on complex procedures for chemical synthesis, availability and purity of chemical solvents and reagents, the use of high-vacuum systems, sophisticated considerations of molecular stereochemistry, physicochemical properties of solvents and solutes, the reproducibility of tests for biological activity, the nature of the mechanism of drug action, and all the unforeseen problems and challenges that invariably occur during basic scientific research and development.
V. Opium and the Opiates
It is clear from legislative efforts that opium has a long colorful history of use in many societies dating back many hundreds of years. In the beginning, opium use was well accepted, but as the liabilities of chronic usage became increasing apparent, the drug fell into disfavor.13 Opium is obtained from the seeds of the opium poppy plant which contains over 25 alkaloid-type chemical compounds. Morphine is the principle opiate alkaloid present in the plant, about 10%, and codeine is present at about 0.5%. Two nonopiod alkaloids found in the plant, thebaine and papaverine, have turned out to be useful in the synthesis of other drugs. The extraction of morphine from crude opium at the beginning of the 19th century began the modem era of drug treatment. This isolation of a pure and powerful compound allowed reproducible and quantitative drug treatment in humans in place of the unreliable results obtained from natural unextracted sources. Synthetic drug development began in the mid 19th century, primarily in Germany, while development of a modern technological drug industry in the U.S. began after World War I. By the 1940s the technology was so developed as to permit highly specific molecular modification of compounds in order to alter potency and toxicity of the new synthetic drugs.

Figure 3.
Molecular structures for the opiate drugs morphine, methadone, and meperidine.
The chemical structures for examples from the major classes of opiate drugs are shown in Figure 3. Morphine is a phenanthrene type of molecular structure, while methadone, a synthetic drug used in the treatment of opiate addiction, is a member of the phenylheptylamine class of opioid compounds and meperidine, a synthetic drug known as Demerol®, is of the phenylpiperidine class. These chemicals are all in Schedule II as they have medical use and a high potential for abuse that can lead to addiction. The use of slight structure changes to alter pharmacological activity applies to the opiate drugs perhaps more dramatically than for the benzodiazepines. Extensive research has yielded a large number of narcotic agonists and antagonist molecules with a wide range of pharmacological properties as part of the ongoing search for a nonaddicting analgesic drug.
A slight change in the structure of the morphine molecule results in a great change in activity. Simply by replacing one of the H atoms of the CH3 group shown in the top right portion of structure in Figure 3 with the group -CH=CH2 the morphine molecule, which produces a strong analgesic effect, is transformed into the nalorphine molecule which is a strong antagonist that completely blocks the effects of morphine. Replacement of the H atoms of the two OH groups by acetyl groups, CH3CO gives the structure of heroin. With this slight change in the molecular structure the drug is more lipophilic than morphine and thus, crosses the blood-brain barrier more easily into the brain and produces an enhanced pharmacologic effect that is readily recognized by experienced opiate users. In a similar manner for methadone, a synthetic opiate used in replacement therapy for treatment of heroin addiction, a slight change to the structure produces the widely used drug propoxyphene or Darvon®. Such slight changes produce what are often called look-alike drugs.
Meperidine, shown in Figure 3, is a Schedule II controlled substance that has been a template for the design of a look-alike drug not on the controlled substances list. A slight change in the structure gives the drug known as MPPP, a Demerol® look-alike drug which is known on the streets as synthetic heroin. The structure differs from meperidine only in that the group bonded to the ring, -COO, is altered to -OCO in MPPP. It is now known that MPPP usually includes an impurity MPTP that has been found to produce permanent brain damage that creates the symptoms of Parkinson's disease.14 Extensive research has lead to a full understanding of the lethal results of consuming this street drug. The detailed story of the scientific pursuit and identification of the cause and the manner in which this occurs is the topic of Chapter 15.
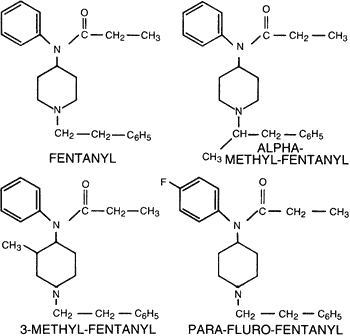
Figure 4.
Molecular structures for fentanyl and analogues.
Another opiate that has created serious health hazards on the streets is fentanyl, or Sublimaze®, which is a potent, extremely fast-acting narcotic analgesic that subsequently has a high abuse liability. It is approximately 100 times more potent than morphine and has been a template for many look-alike drugs for the clandestine chemist to synthesize new uncontrolled substance analogues. Figure 4 shows the molecular structures for fentanyl and the analogues alpha-methyl-fentanyl, 3-methyl-fentanyl, and para-fluoro-fentanyl. While it is clear that the structural changes are very slight, the change in pharmacologic activity is dramatic. On the basis of classic animal tests to evaluate relative potencies, the analogue para-fluoro is 100, alpha-methyl is 900, and 3-methyl is 1100 times more potent than morphine. Statistics gathered by the DEA suggest that drug overdose deaths associated with fentanyl-like drugs has increased steadily over the last 5 years, with a total of 50 deaths reported from California alone.15 The National Narcotic Act of 1984 has been invoked to rapidly classify a number of these new compounds as controlled substance analogues of fentanyl.
VI. The Hallucinogens
It may be appropriate that two of the popular street drugs of this type go by such names as "Adam" and "Eve" since they produce pharmacological effects by interfering with the basic neurotransmitter system for the autonomic nervous system which regulates bodily function and response. Unlike the opiates, where there is substantial understanding of the biological receptors involved in opiod pharmacological response, there is much less understanding of the catecholamines and receptors for the sympathetic nervous system. There is detailed knowledge about the molecular nature of the chemical transmitters, however, and structure-activity studies are providing information about receptor structure and function as well.
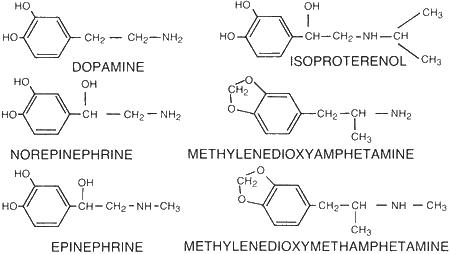
Figure 5.
Molecular structure for dopamine, norepinephrine, epinephrine,
isoproterenol, 3,4-methylenedioxyamphetamine (MDA), and
3,4-methylenedioxymethamphetamine (MDMA).
Amino acids are used by the body to synthesize dopamine which is then transported into the nervous system neuronal vesicles where it undergoes biotransformation to form norepinephrine, the basic adrenergic neurotransmitter involved in the control and regulation of such cardiovascular and peripheral functions as heart rate, blood flow, blood vessel dilation and contraction, and muscle constriction and relaxation. Figure 5 shows the chemical structures for dopamine, norepinephrine, and epinephrine, another of the neurotransmitters. These three compounds are all natural or endogenous compounds which, when administered as a treatment drug, produce pharmacological effects by direct interaction with adrenergic neuron receptors. The synthetic drug isoproterenol, also shown in Figure 5, is also a direct-acting catecholamine. The three catecholamine compounds - norephinephrine, ephinephrine, and isoproterenol — have clinical use based on their actions on bronchial smooth muscle, blood vessels, and the heart. Because of their cardiovascular and central nervous system actions they can also have serious side effects if used improperly. Also shown in Figure 5 are the chemical structures for methylenedioxyamphetamine or MDA and methylenedioxymethamphetamine or MDMA. They clearly have similarities in chemical structure to the catecholamines and probably have similarities in pharmacological actions as well.

Figure 6.
Chemical structures for amphetamine, methamphetamine, phenyl-
propanolamine, hydroxyamphetamine, ephedrine, and phenylephrine.
To explore briefly the complexity of action of this type of drugs, some amphetamine-type drugs are presented in Figure 6 which are closely related to the catecholamine class. It is known that amphetamine does not act directly on the adrenergic receptor, but is taken up into the neuronal vesicle where it displaces norepinephrine. This indirect-acting drug elicits sympathomimetic effects on the central nervous system, stimulation or mood elevation, and on the peripheral vasculature, increased blood pressure and heart rate. All the drugs in Figure 6, except phenylephrine, are indirect-acting agents due to the fact that their action depends on displacement of stored norepinephrine. When the mechanism of action is in this indirect fashion, the pharmacologic response under repeated administration of a given dose produces tachyphylaxis, which is a gradually diminishing response to the drug stimulus also called drug tolerance. The slight structural difference between amphetamine and methamphetamine, which is seen in Figure 6 as the replacement of an H with a CH3 on the nitrogen center, is sufficient to allow the latter to cross the blood-brain barrier more easily and elicit a stronger central nervous system stimulus, while also having a weaker peripheral action. Therefore, the ratio of central-to-peripheral action is altered by the slight change in the structure of the drug molecule. Ephedrine, with the added hydroxy group OH, also has a different ratio of central-to-peripheral action than amphetamine as its central effect is weaker, while its peripheral action is prolonged in duration. Phenylpropranolamine and hydroxyamphetamine, which also have slight structural alterations from amphetamine, are similar in structure to the direct-acting catecholamines, but are indirect-acting drugs. Both are less potent central nervous system stimulants than ephedrine, while they have a marked effect on peripheral blood vesicles. The time course of effect for phenylpropanolamine is probably biphasic in nature as it first causes vasoconstriction which is then followed by vasodilation, thus producing first a pressor and then a depressor action on mean blood pressure. To further stress the complexity of this class of drugs, phenylephrine is a direct-acting sympathomimetic drug, but it has very little central action.
It should be clear that this general class of drugs are extremely complex as to the nature of their pharmacological effects in the human body.16 As with all drugs, they can also produce adverse effects. This toxicity for drugs that act on the cardiovascular and central nervous system can produce a suddenly life-threatening crises, especially for persons that have a predisposition or an undetected vulnerability.
MDA has been a Schedule I drug since the Controlled Substances Act of 1970, while MDMA was put on the controlled substances list in 1985 by the emergency provisions of the 1984 National Narcotics Act. This was a move that caused controversy as there are claims that MDMA has value in psychotherapy and research will be greatly impeded by Schedule I classification. A detailed consideration of MDMA, its pharmacology, and related issues is presented in Chapter 14.
VII. Conclusions
For the street drug there is no assurance of proper preparation of pure drug and there is no information available on controlled clinical studies of their safety. Furthermore, persons consuming such drugs are probably not adequately aware of the potential risks produced by the pharmacological action to their body. Therefore, the unknown consequences of consumption must truly be considered a hazard to health. The recent report that MDMA may be related to deaths due to heart-related causes must be seriously considered.17 And the new arrival to the street scene of methylenedioxyethamphetamine or MDEA, alias "Eve", just one more new drug on the street, again demands that we reflect on the nature of our social condition. What are the reasons for the popularity of chemical escape from reality? It cannot be as simplistic an answer as "because it feels good". We must find out why there are among us so many humans for whom it is considered a necessary ingredient to life. Attempts to control the supply of illicit drugs will not solve the problem. In a nation where pharmaceutical sales are in the billion-dollar range, one must conclude that drugs have a prominent position in society, for treatment as well as for recreation/abuse. the lack of general knowledge of the populus concerning the basic concepts of pharmacology is widely prevelant and dangerous. To provide basic education and information on how the body handles a drug and how the drug effects the body will provide a positive step of great import towards informed consent and awareness before drug consumption, thus assuring better medical therapy and perhaps less drug abuse.
References
- Krantz, J. C., Jr., Historical Medical Classics Involving New Drugs, Williams & Wilkins, Baltimore, 1974, 121.
- LaWall, C. H., Four Thousand Years of Pharmacy, Lippincott, Philadelphia, 1927, chap. 1.
- Ziporyn, T., A growing industry and menace: makeshift laboratory's designer drugs, J. Am. Med. Assoc., 256, 3061 (1986)
- Rangel, C. B., Statement, Designer Drugs, Hearing before the Committee On the Budget, United States Senate, Washington, D.C., July 18, 1985, 6.
- Pharmaceutical Manufacturers Association, Annual Report, Washington, D.C., 1986.
- Jaffe, J. H., Drug addiction and drug abuse, The Pharmacological Basis of Therapeutics, 7th ed., Gilman, A. G., Goodman, L. S., Rail, T. W., and Murad, F., Eds., New York, MacMillan, 1985, chap. 23.
- Rapaka, R. S., Barnett, G., and Hawks, R., Eds., Opioid Peptides: Medicinal Chemistry, National Institute on Drug Abuse, Rockville, Md., 1986.
- Barnett, G., Trsic, M., and Willette, R., Eds., QuaSAR: Quantitative Structure Activity Relationships of Analgesics, Narcotic Antagonists, and Hallucinogens, NIDA Research Monograph, No. 22 (1978), Rockville, Md.
- Soine, W. H., Clandestine Drug Synthesis, Med. Res. Rev. 6, 41 (1986)
- Rexed, B., Edmondson, K., Khan, I., and Samsom, R. J., Eds., Guidelines for the Control of Narcotic and Psychotropic Substances, World Health Organization, Geneva, 1984.
- Harvey, S. C., Hypnotics and Sedatives, in The Pharmacological Basis of Therapeutics, 7th ed., Gilman, A. G., Goodman, L. S., Rail, T. W., and Murad, F., Eds., Macmillan, New York, 1985, chap. 17.
- Trevor, A. J. and Way, W. L., Sedative hypnotics, in Basic and Clinical Pharmacology, Katzung, B. G., Ed., Lange Medical Publications, Los Altos, Calif., 1984, chap. 20.
- Way, E. L., History of opiate use in the Orient and the United States, in "Opioids in Mental Illness: Theories, Clinical Observations, and Treatment Possibilities", Verebey, K., Ed., Ann. N.Y. Acad. Sci. 398, 12 (1982)
- Snyder, S. H., and D'Amato, R. J., MPTP: a neurotoxin relevant to the pathophysiology of Parkinson's disease, Neurology, 36, 250 (1986)
- Henderson, G., Statement in Designer Drugs, Hearing before the Committee On The Budget, United States Senate, Washington, D.C., July 18, 1985, 22.
- Robinson, R. L., Sympathomimetic drugs, in Modern Pharmacology, Craig, C. R. and Stitzel, R. E., Eds., Little, Brown, Boston, 1982, 23.
- Dowling, G. P., McDonough, E. T., III, and Bost, R. O., "Eve" and "Ecstasy": a report of five deaths associated with the use of MDEA and MDMA, J. Am. Med. Assoc. 257, 1615 (1987)





