Since the introduction of flash chromatography1, several modifications to the apparatus and the pressure source used in the technique have been published2–7. The most widely used method is to force a solvent mixture from a reservoir on top of the column through the column, using pressurized air. A lateral reservoir attached to the side of the column has also been described8. There are two problems associated with an overhead reservoir. The main hazard is adding and keeping large volumes of solvents above the column, and usually above the head of the laboratory worker. Furthermore, there is also no easy way to change the composition of the solvent mixture during the chromatographic process and often large volumes of solvents have to be discarded after the separation is completed.
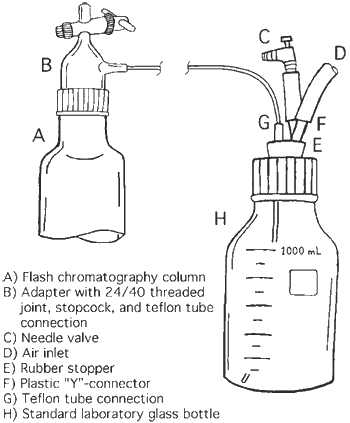
Figure 1. Flash chromatography apparatus.
To solve these problems we have developed a new method for performing flash chromatography in which the solvent reservoir is at the side of the column, in a separate bottle, connected with a Teflon tube to an adapter for standard flash chromatography columns. The design is outlined in Figure 1. The adapter (B) is similar to the standard flow controller and consists of a 24/40 joint with a threaded screw-cap to fit standard flash chromatography columns (A), a stopcock for quick pressure release, and a Teflon tube connection. The solvent reservoir is made from a standard glass laboratory bottle (H) with a hole drilled in the cap to fit a rubber stopper (E). A shallow groove can be cut around the stopper for enhanced fitting. Two holes are drilled in the rubber stopper to insert a glass Teflon tube connection (G) and a plastic Y-connector (F). The pressurized air tube (D) is connected to one arm of the Y-connector, and a needle valve (C) for fine-tuning of the pressure is attached to the other arm via a short piece of rubber tubing.
The sample is applied on top of the column and the adapter is fastened while leaving the stopcock open. The preferred solvent mixture is added to the bottle and a slight pressure is applied, forcing the solvent mixture into the column. The rate of solvent addition is adjusted so as not to disturb the silica bed. The stopcock is closed when the column is filled to an appropriate level and the pressure is adjusted, using the needle valve, to give a suitable flow of the eluent. The solvent can easily be changed or replenished during the separation by simply changing bottles. When the separation is completed the bottle is removed and only a small amount of solvent, left in the adapter, is wasted. We have used this methodology for hundreds of separations and found it to be safe and reliable. It is also convenient to store different solvent mixtures in bottles ready to use, avoiding unsafe solvent handling.