Distillation, recrystallization, and extraction are all important techniques for the purification of organic compounds. But the technique used most commonly in modern organic research is "flash" chromatography. In traditional column chromatography a sample to be purified is placed on the top of a column containing some solid support, often silica gel. The rest of the column is then filled with a solvent (or mixture of solvents) which then runs through the solid support under the force of gravity. The various components to be separated travel through the column at different rates and can then be collected separately as they emerge from the bottom of the column. Unfortunately, the rate at which the solvent percolates through the column is slow. In flash chromatography however air pressure is used to speed up the flow of solvent, dramatically decreasing the time needed to purify the sample.
I have included excerpts from an article in the Journal of Organic Chemistry that describes the technique. As mentioned below, an appropriate solvent system for your flash column is one that produces an Rf on TLC of approximately 0.35-0.4. The columns available in the organic lab have a diameter of approximately 20 mm, so are useful for purifying 100-400 mg of sample (see table below).
Excerpted from:
Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution
W.C. Still, M. Kahn, and A. Mitra
J. Org. Chem. 43, 2923-2925 (1978)
We wish to describe a simple absorption chromatography technique for the routine purification of organic compounds. Large scale preparative separations are traditionally carried out by tedious long column chromatography. Although the results are sometimes satisfactory, the technique is always time consuming and frequently gives poor recovery due to band tailing. [...] We have recently developed a substantially faster technique for the routine purification of reaction products we call flash chromatography. Although its resolution is only moderate (ΔRf ~0.15 on TLC), the system is extremely inexpensive to set up and operate and allows separations of samples weighing 0.01-10.0 g in 10-15 min.
Flash chromatography is basically an air pressure driven hybrid of medium pressure and short column chromatography. [...] Column performance is quite sensitive to the rate of elution and is best with relatively high eluant flow rates. The solvent head above the absorbent bed should drop 2.0 in./min for optimum resolution.
A detailed procedure is presented in the experimental section and is summarized as follows: (1) A solvent is chosen which gives good separation and moves the desired component to Rf = 0.35 on analytical TLC. (2) A column of the appropriate diameter (see Table I) is selected and filled with 5-6 in. of dry 40-63 µ silica gel. (3) The column is filled with solvent and pressure is used to rapidly push all the air from the silica gel. (4) The sample is applied and the column is refilled with solvent and eluted at a flow rate of 2 in./min.
Table I
Typical Parameter Values
Column Diameter | Eluanta
| Sample Loading | Fraction Size |
|
ΔRf ~0.2 | ΔRf ~0.1 | |||
10 mm |
100
mL |
100 mg | 40 mg | 5 mL |
20 mm |
200
mL |
400 mg | 160
mg | 10 mL |
30 mm |
300
mL |
900 mg | 360
mg | 20 mL |
40 mm |
600
mL |
1600 mg | 600
mg | 30 mL |
50 mm |
1000 mL
|
2500 mg | 1000
mg | 50 mL |
a Typical volume required for packing and elution.
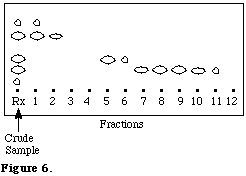
The time required to elute the desired components from the column is generally so fast (5-10 min) that we have abandoned automatic fraction collectors in favor of a simple rack holding test tubes. Small fractions are typically collected early in the elution with larger ones being collected toward the end of the chromatography. Separated components are conveniently detected by spotting ~5 mL of each fraction along the long side of 7x2.5 cm TLC plate and then by developing them sideways. Heavier spotting may be required for small samples or highly retentive components. A typical separation is shown in Figure 6.
Experimental
Flash Chromatography. General Procedure.
First a low viscosity solvent system (e.g. ethyl acetate/30-60°C petroleum ether) is found which separates the mixture and moves the desired component on analytical TLC to an Rf of 0.35. If several compounds are to be separated which run very close on TLC, adjust the solvent to put the midpoint between the components at Rf = 0.35. If the compounds are widely separated, adjust the Rf of the less mobile component to 0.35. Having chosen the solvent, a column of the appropriate diameter (see text, Table I) is selected (We will be using columns of 20 mm diameter.) Dry 40-63 µm silica gel is poured into the column in a single portion to give a depth of 5.5-6 in. With the stopcock open, the column is gently tapped vertically on the bench top to pack the gel. Next a 1/8 [to 1/4] in. layer of sand is carefully placed on the flat top of the silica gel bed and the column is clamped for pressure packing and elution. The solvent chosen above is then poured carefully over the sand to fill the column completely. [Air pressure is then applied by holding the cork containing the air line on the top of the column.] This will cause the pressure above the absorbent bed to climb rapidly and compress the silica gel as solvent is rapidly forced through the column. It is important to maintain the pressure until all the air is expelled and the lower part of the column is cool; otherwise, the column will fragment and should be repacked unless the separation desired is a trivial one. The pressure is then released and excess eluant is forced out of the column. The top of the silica gel should not be allowed to run dry. Next the sample is applied by pipette as a 20-25% solution in the eluant to the top of the adsorbent bed and the flow controller is briefly placed on top of the column to push all of the sample into the silica gel. The solvent used to pack the column is ordinarily reused to elute the column. The walls of the column are washed down with a few milliliters of fresh eluant, the washings are pushed onto the column as before, and the column is carefully filled with eluant so as not to disturb the adsorbent bed. [The cork is held securely onto the top of the column and the air regulator is carefully adjusted to cause the surface of the column to fall 2.0 in./min.] Fractions are collected until all the solvent has been used (see Table I to estimate the amount of solvent and fraction size). It is best not to let the column run dry since further elution is occasionally necessary. Purified components are identified by TLC and the appropriate fractions are combined and the solvent removed by rotary evaporation to yield the desired material. If the foregoing instructions are followed exactly, there is little opportunity for the separation to fail.