II. Synthesis Routes
Scheme 1
Reductive amination
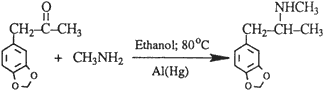
A. The Reductive Amination Route
The most frequently used method to prepare MDMA in The Netherlands can be described as a low pressure reductive amination at slightly elevated temperatures3,4 (Scheme 1).
The structural information and eight-peak MS data of the impurities that are reported for this route of synthesis are summarized in Table 1. The compounds given in the table include starting materials and their impurities, hydrogenated compounds originating from starting materials, and nitrogen-containing compounds - intermediate substances resulting from the reaction of phenylpropanone with impurities in methylamine such as ammonia and higher alkylated amines.
Scheme 2
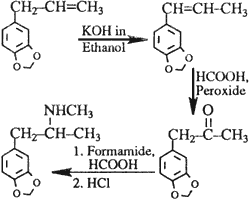
Leuckart reaction
Besides these impurities relating to chemical synthesis and the substances used in it, the MDA and the MDMA preparations can be contaminated by a host of strange compounds4 including caffeine, cocaine, ketamine, quinine, and amphetamine; the latter substance also contains typical impurities aziridines, pyrimidines, and di-(β-phenylisopropyl)amine.
B. The Leuckart Reaction
This reaction is seldom used for the synthesis of the substituted amphetamines4,5. Using safrole as a starting compound in order to produce the phenylpropanone, the reaction can be schematically depicted as shown in Scheme 2.
The structural information and eight-peak MS data of the impurities that are reported for this route of synthesis are summarized in Table 2. Again, many impurities derive from starting materials and accompanying chemicals; others originate from condensations between the starting material and the end product.
Scheme 3
Nitropropene route
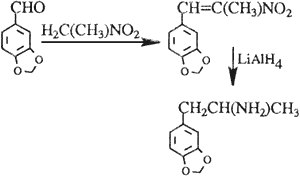
C. The Nitropropene Route
The condensation reaction (Scheme 3) between nitroethane and piperonal has been adopted for the production of MDA5,6.
The structural information and eight-peak MS data of the reported impurities that are known for this route of synthesis are summarized in Table 3. Again, the presence of impurities in the starting materials was noticed, while the most of the other impurities found can best be explained by assuming condensation reactions between starting materials, intermediate, and final products.
D. The Bromopropane Route
Scheme 4
Bromopropane route
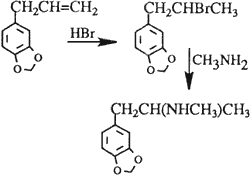
The reaction of safrole (obtained from sassafras oil) with hydrobromic acid shown in Scheme 4 was intensively studied7,8.
All bromination products of the other essential oils associated with the starting chemical safrole can be found in MDA or MDMA preparations, depending on the extent of purification attained by the individuals that are producing the illicit drugs. The structural information and eight-peak MS data of the impurities that are reported for this route of synthesis are summarized in Table 4. The impurities given here refer to various compounds present in safrole; the brominated products of these substances resulting from the reaction of different compounds in safrole with hydrobromic acid; and the amino compounds originating from the amination of the bromine-containing substances.