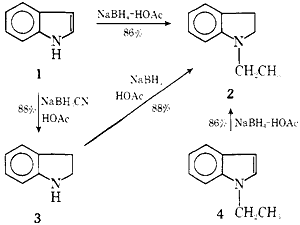
We wish to report that sodium borohydride (NaBH4) in neat carboxylic acids sequentially reduces the indole double bond and alkylates the nitrogen atom to give N-alkylindolines, e.g., 1 → 2, and that this combination of reagents conveniently alkylates primary and secondary aromatic amines, e.g., 3 → 2 (Scheme I).
Scheme I.
Transformations in Acetic Acid
Although the reduction of indoles to indolines has received considerable attention,2 there is no general, efficient procedure for this transformation. Encouraged by the tendency of the indole ring to protonate at the 3-position3,4 and by the observations that enamines can be reduced by NaBH4 in acetic acid-tetrahydrofuran (HOAc-THF)5 and sodium cyanoborohydride (NaBH3CN),6 we have examined the behavior of indoles with NaBH4 in neat carboxylic acids.
Quite unexpectedly, the reaction of indole (1) with NaBH4 in glacial HOAc gives N-ethylindoline (2) in 86% yield. Likewise, the reactions of indoline (3) and N-ethylindole (4) with NaBH4-HOAc give 2 in high yield.
This unprecedented reduction-alkylation of indoles and reduction of N-alkylindoles7 with NaBH4 in liquid carboxylic acids appears to be a general transformation (Table I). However, the stronger acid, formic, produces indole dimers and other products in addition to N-methylindoline. Interestingly, the reaction of 1 with NaBH4 in trifluoroacetic acid (CF3CO2H) gives indoline (3), the product of reduction without alkylation, in low yield. The yield of 3 can be increased to 88% when 1 is treated with NaBH3CN-HOAc (Table I). This latter reaction permits a very convenient synthesis of 3.
Scheme I.
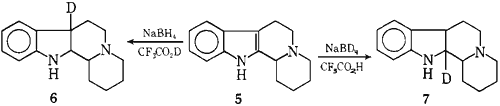
We believe that the reaction of 1 → 2 involves 3-protonation of indole,4 followed by reduction of the resulting indolenium ion to give 3,8 which is subsequently alkylated (vide infra). The reduction of the indoloquinolizidine alkaloid 5 with NaBH4-CF3CO2H proceeds without alkylation (72%) and the deuteration experiments, 5 → 6 and 5 → 7, are in accord with our suggested mechanism.
The combination NaBH4-RCO2H also provides for the facile alkylation of a variety of primary and secondary aromatic amines9 (e.g., 3 → 2), and the reaction can be controlled to give mono- or dialkylation of primary amines. Thus, aniline with NaBH4-HOAc at 20°C gives N-ethylaniline, and further reaction at 60°C gives N,N-diethylaniline (Table II).
This alkylation method is extended by our observation that aldehydes and especially ketones are reduced to alcohols relatively slowly by NaBH4-RCO2H,10 so that unsymmetrical tertiary amines can be prepared from primary amines in one flask. Thus, aniline with NaBH4-HOAc-acetone gives either N-isopropylaniline or N-ethyl-N-isopropylaniline, depending on the temperature (Table II). This versatility is not available with previous methods for reductive amination6,11 of aldehydes and ketones (H2,11a,b HCO2H,11c,d NaBH3CN,6,11e NaBH4,11f,g Fe(CO)511h)
We view the amine alkylation as a stepwise process: (1) reduction of carboxylic acid to aldehyde12 (or aldehyde equivalent), perhaps via one or more acyloxyborohydride species13 and intra- or intermolecular hydride reduction of the carbonyl group; (2) reaction of the aldehyde with amine to form an iminium ion; and (3) hydride reduction14 of the iminium ion to product amine.
Although amides are side products in some cases, they are very clearly not obligatory intermediates in the alkylation reaction. Thus, N-acetylindoline and N-acetylindole are recovered in 67% and 82% yield, respectively, after treatment with NaBH4-HOAc, under conditions which convert indoline completely into N-ethylindoline. Likewise, it seems unlikely that diborane is the reducing agent in the reaction, since externally generated gaseous diborane bubbled into amine-HOAc gives clean acylation and not alkylation of the amine.15
| Substrate | Carboxylic acid | Productb | Yieldc |
1 | HOAc | N-Ethylindoline | 86% |
| HCOOH | N-Methylindoline | 53% | |
| CH3CH2CO2H | N-n-Propylindoline | 69% | |
| (CH3)2CHCO2H | N-Isobutylindoline | 49% | |
| CF3CO2H | Indolined | 36% | |
| HOAc-NaBH3CN | Indoline | 88% | |
| 2-Methylindole | HOAc |
1-Ethyl-2-methylindoline | 84% |
| 3-Methylindole | 1-Ethyl-3-methylindoline | 45% | |
| 2,3-Dimethylindole | 1-Ethyl-2,3-dimethylindoline | 60% | |
| Tetrahydrocarbazole | N-Ethylhexahydrocarbazole | 77% | |
| 7-Methylindole | 1-Ethyl-7-methylindoline | 90% | |
| N-Methylindole | N-Methylindoline | 86% | |
| N-Ethylindole | N-Ethylindoline | 86% | |
| 1,2-Dimethylindole | 1,2-Dimethylindoline | 84% |
- Conditions are typically 0.005-0.02 mol of indole dissolved in
30-150 mL of dry carboxylic acid at 15-20°C to which is slowly
added 0.02-0.20 mol of NaBH4 pellets (Alfa Inorganics, Inc.).
A brief heating period at 50-60°C is sometimes necessary to
complete the reaction. Reactions can be monitored by TLC or UV. - Identified by comparison with authentic material or by conversion
to known derivatives (picrate. methiodide). All products
exhibited satisfactory ir, nmr. and uv spectra. - Yields are for pure distilled material. In most cases starting
indole was the only other material present in the reaction mixture.
The reactions have not been optimized. - A small amount of N-trifluoroethylindoline is also formed.
| Substrate | Carboxylic acid |
Productb | Yieldc |
Aniline | HOAc | N-Ethylaniline | 88% |
HOAc (50-60°C) | N,N-Diethylaniline | 74% | |
HOAc-Me2CO | N-Isopropylaniline | 68% | |
HOAc-Me2CO (50-60°C) | N-Ethyl-N-isopropylaniline | 79% | |
HOAc-PhCHO (50-60°C)d | N-Benzyl-N-ethylaniline | 80% | |
(CH3)3CCO2He | N-Neopentylaniline | 80% | |
N-Methylaniline | HOAc | N-Ethyl-N-methylaniline | 72% |
HOAc-Me2CO | N-Isopropyl-N-methylaniline | 78% | |
HCO2H | N,N-Dimethylaniline | 77% | |
HOAc-(HCHO)n-THF | N.N-Dimethylaniline | 59% | |
CH3CH2CO2H | N-Methyl-N-propylaniline | 83% | |
N-Ethylaniline | CH3CH2CO2H | N-Ethyl-N-propylaniline | 70% |
N-Isopropylaniline | HOAc | N-Ethyl-N-isopropylaniline | 69% |
Indoline | HOAc | N-Ethylindoline | 88% |
CF3CO2H | N-Trifluoroethylindolinef | 7%g | |
Diphenylamine | HOAc | N-Ethyldiphenylamine | 80% |
Carbazole | HOAc | N-Ethylcarbazole | 92% |
5H-Dibenz[b,f]azepine | HOAc | 9-Ethyl-5H-dibenz[b,f]azepine | 72% |
- + b. + c. See corresponding footnotes in Table 1.
- N-Benzylaniline could be isolated from the 20°C reaction.
- his reaction was also run with diglyme as a cosolvent.
- Indoline was recovered in 51% distilled yield.
- From the pot residue there was isolated in 34% yield an indoline dimer containing three trifluoroethyl groups.