Abstract
An improved two-layer method of the Nef reaction which is effective for the conversion of a variety of aromatic nitroalkanes into carbonyl compounds is described.
The elongation of aldehydes and the elongating transformation of aldehydes to ketones have been useful tools in organic synthesis. A useful method for such transformations is the combination of the preparation of nitroalkenes from aldehydes by the Knoevenagel condensation and subsequent conjugate reduction followed by conversion of the resulting nitroalkanes into carbonyl compounds. Although this conversion method for aliphatic aldehydes has already been established,1 virtually no applicable methods for aromatic aldehydes is known due to the difficulties involved in the reduction of aromatic nitroalkenes to the corresponding nitroalkanes (1).3 In addition, despite much work in this field, there is very little data concerning the convertibility of aromatic nitroalkanes (1) into the corresponding carbonyl compound (3). This is probably due to the same reason.
Table I
Carbonyl Compounds (3)
from Nitroalkanes (1)
Product |
Ar |
R |
Yield* |
3a | Ph- | H- | 64% |
3b | p-Cl-Ph- | H- | 36% |
3c | p-MePh- | H- | 65% |
3d | p-MeOPh- | H- | 67% |
3e | Veratryl- | H- | 66% |
3f | 2-Furyl- | H- | 47% |
3g | α-Naphtyl- | H- | 54% |
3h | Piperonyl- | H- | 29% |
3i | Ph- | Me- | 89% |
3j | Ph- | Et- | 91% |
3k | Ph- | n-Pr- | 93% |
* Isolated, pure products.
Although several current methods2 are available for the conversion of common nitroalkanes into the corresponding aldehydes and ketones, a more useful method for these transformations is the Nef reaction. The original Nef reaction involves the solvolysis of alkali nitronates (i.e. aci-nitroalkanes) with aqueous or alcoholic acid solution. However, we have found that the conversion of 1 into 3 by the original method gave low yields of the products due to the unusual instability of the carbonyl products (3) in acid solution.
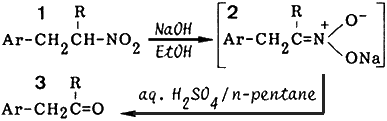
In a preceding paper,3 we reported a convenient and efficient method for the preparation of aromatic nitroalkanes from aromatic nitroalkenes by the reduction using o-phenylenediamine and benzaldehyde. In this paper, we will describe an improved two-layer method of the Nef reaction. It is effective for the conversion of aromatic nitroalkanes (1) into the corresponding carbonyl compound (3).
Our method involves the addition of an aqueous solution of sodium nitronates (2) to a two-layer solution of the acid and n-pentane. The improved Nef reaction was carried out on 11 selected nitroalkanes. The results in Table I show that the reaction can be effectively applied to a variety of aromatic nitroalkanes. This two-layer method enscones the product in the pentane layer and avoids contact of the product with the acid. This modification efficiently diminishes any side reactions as evident from the high yields of product.
Experimental
General Procedure for the Preparation of Carbonyl Compounds (3) from Nitroalkanes (1):
A solution of nitroalkane (1) (5 mmol) in absolute ethanol (10 ml) was added dropwise to a solution of NaOH (0.8 g, 20 mmol) in ethanol (10 ml) under nitrogen at room temperature. The mixture was stirred for 5 min and the solvent was evaporated at room temperature under reduced pressure. A solution of the resulting salt (2) (sodium nitronate) in water (20 ml) was slowly added dropwise to the two-layer mixture of sulfuric acid (conc. H2SO4 2.5 ml/water 24 ml) and n-pentane (20 ml) with stirring under ice-water cooling. After the addition, stirring was continued for 1 hr at 0°C and the pentane layer was separated out. The aqueous layer was then extracted with methylene chloride. The combined pentane solution and the extract was dried with anhydrous sodium sulfate and then filtered through sillca gel (Wakogel C-300). Removal of the solvent gave almost pure product (3).