Abstract
Application of ultrasound has been found to greatly assist the Knoevenagel aldol condensation reaction of activated methylenes with aromatic aldehydes under mild conditions. The outcome of the ultrasound-promoted reaction depends upon the electronic nature of the aromatic aldehyde, the solvent employed and the addition of acids, bases or ammonium salts.
The Knoevenagel condensation reaction of carbonyl-containing compounds with active methylenes is a classic general method for the preparation of valuable synthetic intermediates. Examples include the condensation of aromatic aldehydes 1 with active methylenes such as malonic acid or β-ketoacid derivatives, allowing access to cinnamic acid derivatives 2 and cinnamyl ketones 3, while the use of nitroalkanes (Henry reaction) as the methylene component1 leads to nitroaldol products 4 or nitroalkenes 5.
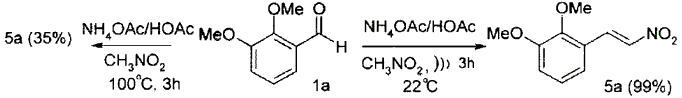
A multitude of promoters have been developed for these reactions including acids, bases and ammonium salts. Typical conditions to effect the condensation of nitroalkanes with aromatic aldehydes consist of heating an acetic acid solution of the aldehyde with the appropriate nitroalkane with ammonium acetate at 100°C for a few hours.2 In this way nitroalkenes 5 may be isolated in moderate to high (30-95%) yield depending on the aldehyde used. We have observed lower yields of nitroalkenes result from the condensation of electron rich aromatic aldehydes, a result that may be general.3 For example, under these standard Henry conditions, 2,3-dimethoxybenzaldehyde 1a condensed with nitromethane to give the nitroalkene 5a in only 35% yield, the mother liquors being contaminated with a resinous non-crystalline material that we attributed to phenol-formaldehyde type polymerization. Recent reports concerning the ultrasound promoted elimination of HI from nitroalkanes to yield nitroalkenes4 and ultrasound promoted carbonyl addition reactions5,6 led us to consider the application of ultrasound as a low temperature promoter of the above Henry reaction. Repetition of the reaction of 1a with the same reagents but at room temperature and application of ultrasound led to a rapid, clean condensation (complete in 3h) and subsequent isolation of the nitroalkene product 5a in 99% yield, with no resinous side products being produced.
| Starting Aldehyde | Yield | Method |
| 2,3-Dimethoxybenzaldehyde | 99% | A |
| 3,4-Methylenedioxybenzaldehyde | 99% | A |
| 2,4,6-Trimethoxybenzaldehyde | 85% | A |
| 4-Acetamidobenzaldehyde | 95% | A |
| 4-Methylthiobenzaldehyde | 96% | A |
| Indole-3-carboxaldehyde | 93% | A |
| 3-Methoxy-4-hydroxybenzaldehyde | 89% | A |
| 4-Chlorobenzaldehyde | 70% | B |
| 4-Nitrobenzaldehyde | 61% | B |
| 3-Nitrobenzaldehyde | 51% | B |
No reaction occurs under these conditions at room temperature until ultrasound is applied. In addition, attempts to conduct the ultrasound promoted reaction at room temperature without acetic acid, or without ammonium acetate, failed. Other studies using primary or secondary amines in place of ammonium acetate were not as successful. The ultrasound promoted reaction proved to be general (Table 1) for a variety of electron rich aromatic aldehydes under these conditions. High yields of nitroalkenes were isolated for all alkoxy-substituted aromatic aldehydes investigated. Thus, piperonal 1b reacted to give nitroalkene 5b (99%) while the 2,4,6-trimethoxy derivative 1c gave nitroalkene 5c (84%) demonstrating steric factors to be of little detriment. The hetero-substituted aldehydes 1d and 1e and indole-3-carboxaldehyde 1f also produced the corresponding nitroalkenes efficiently, as did vanillin 1g without protection of the phenolic hydroxyl, whereas the aromatic ketones acetophenone and benzophenone failed to react. Electron deficient aromatic aldehydes also reacted under our ultrasound promoted conditions but the major product proved to be the corresponding nitroaldols 4, isolated in poor yield. Modification to the conditions by changing to base catalysis improved the yields to 50-70%. Sonication of a solution of 4-chlorobenzaldehyde 1h according to method B allowed for isolation of the nitroaldol 4h in 70% yield. This method proved general for electron deficient aldehydes. Both 3-nitro and 4-nitrobenzaldehyde gave the corresponding nitroaldols 4i and 4j in good isolated yield.
The application of ultrasound in promoting the Knoevenagel condensation to produce these intermediates offers several advantages over classic refluxing methods. In addition to the general nature of the process described, the lower temperatures employed under ultrasound promotion allow for higher yields of isolated product due to less side reactions such as polymerization occurring. The reactions are also easily scaled-up to multi-gram levels. The lower temperatures are also likely to allow selective condensation in cases involving acid or base labile substrates.
Experimental
Nitromethane and pyridine (Aldrich) were freshly distilled. Ultrasound reactions were performed using a Branson 5510 ultrasound bath or a Crest Tru-Sweep ultrasonic cleaner with little difference.
- Method A:
- A mixture of aldehyde (20.0 mmol), nitromethane (13.0mL), glacial acetic acid (3.3mL) and ammonium acetate (3.324g), sonicated at 22°C for 3h. After removal of nitromethane, partition between dichloromethane and water then brine gave crude product which was recrystallized from aq. ethanol (except 5d, AcOH).
- Method B:
- A solution of aldehyde (1.00 mmol), nitromethane (1.0 mL), ammonium acetate (2.5 mmol) and diisopropylethylamine (0.1 mmol) was sonicated at 22°C for 3-6h, according to TLC. After removal of solvents, work-up as above gave the crude product which was purified on silica gel.