Abstract
Nitration of substituted styrenes by nitryl iodide, followed by treatment of the product with triethylamine, gives β-nitrostyrenes in good yield.
In connection with the search for a more efficient synthesis of 2,3,5-trimethoxyamphetamine1, for use as a drug standard, we have found that nitryl iodide is a particularly effective general reagent for the nitration of substituted styrenes. β-nitrostyrenes are generally prepared by direct nitration of styrenes or by condensation of the corresponding aldehyde with nitroethane2; reduction of the β-nitrostyrenes gives the amphetamine. Nitration of styrene by tetranitromethane is difficult and yields vary greatly. Nitration reagents such as HgCl2-NaNO23 lead to varying degrees of ring nitration as well as the intended reaction at the double bond.
Nitryl iodide is generated in situ by the reaction of silver nitrite with iodine. First reported by Birchenbath in 19324, this reaction was virtually disregarded until 1964. Its synthetic utility and the mild conditions of its application were initially described by Hassner et al.5 Two reports6 on the use of this reagent with unsaturated carbohydrate derivatives appeared later. However, the application of nitryl iodide in general synthesis has not yet been widely explored.
Scheme 1
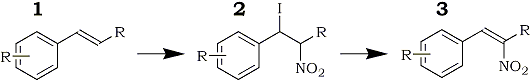
The mechanism of the reaction was examined in detail by Hassner. Nitryl iodide underwent regioselective addition to styrene to form the iodonitro compound (2) which upon treatment with base generated the β-nitrostyrene (3).
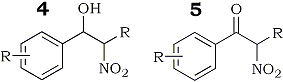
However, contrary to Hassner's claim, we have found that the use of excess iodine does not improve the yield. We deduce that poor yield is attributable to degradation of the unstable iodonitro intermediate which is sensitive to moisture and oxygen. In some instances, hydroxynitro compound (4) and nitroketone (5) could be detected. However, good yields of β-nitrostyrene could be consistently assured by treatment of the crude addition product with triethylamine, immediately following the disappearance of the styrene substrate, as judged by tlc monitoring of the course of the reaction.
Table 1
Nitration of substituted styrenes with nitryliodide.
| Starting Material8 | Solvent |
Yield |
Product8 |
| 2,3,5-Trimethoxy- propenylbenzene |
Ether |
80.3% |
2,3,5-Trimethoxy- phenyl-2-nitropropene |
| 4-Methoxy- propenylbenzene |
THF |
81.8% |
4-Methoxy- phenyl-2-nitropropene |
| Isosafrole | THF |
69.8% |
3,4-Methylenedioxy- phenyl-2-nitropropene |
| Isomyristicin | THF |
53.0% |
β-Nitroisomyristicin9 |
| Propenylbenzene | Ether |
76.4% |
Phenyl-2-nitropropene |
| Styrene | Ether |
45.3% |
β-Nitrostyrene |
The solubility of the β-nitrostyrene product in the reaction medium is a major factor affecting the yield realized: β-nitrostyrene which are relatively insoluble in the reaction medium co-precipitate with the reagent, forming a solid cake which inhibits further reaction. In such a circumstance, changing to a more suitable solvent such as THF is necessary to ensure a respectable yield. Results are summarized in Table 1.
Experimental
A representative nitration was performed as follows:
I2 (1016 mg, 4 mmol) and AgNO2 (616 mg, 4 mmol) were stirred in anhydrous ether (20 ml) at r.t. under nitrogen for 45 min. β-Methylstyrene (236 mg, 2 mmol) and pyridine (632 mg, 8 mmol) in ether were added and the mixture was stirred at r.t. for 3.5 h; after this time the yellow solid was removed by filtration. The filtrate was treated with 0.5 ml Et3N and evaporated to dryness. The residue was then treated with Et3N (1 ml) in CH2Cl2 (1 ml) at r.t. for 1 h. The resulting solution was evaporated to dryness, the residue was dissolved in CH2Cl2, washed with 5% aqueous NaHSO3, 5% aqueous HCl, saturated aqueous NaHCO3 and water, dried over Na2SO4 and solvent removed under reduced pressure to give a dark brown liquid. This material was chromatographed on silica and eluted with 8% ether/hexane to give the pure product (249 mg, 76.4%).