Abstract
Good yields of β-phenethylamine hydrochloride salts are obtained directly from the catalytic reduction of conjugated nitroalkenes in dilute hydrochloric acid.
Reduction of omega-nitrostyrenes (I) is a simple route to the important β-phenethylamines (II). Lithium aluminum hydride has been used successfully for this transformation1-3. While this reagent is admirably suited for small-scale reactions, it is impractical for bulk preparative work because of the hazards inherent in the use of large quantities of this relatively expensive metal hydride and the difficult work-up procedures. In view of these objections, the hydrogenation of I would appear to be a more desirable procedure. This has already been done indirectly, via the oxime, in poor over-all yield4,5 and directly in highly acidic solution6,7. While good yields are reported under the latter conditions, the work-up is tedious.
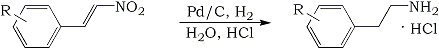
We now wish to report a simple, efficient, and inexpensive method of converting I to II. This procedure, which involves the palladium catalyzed hydrogenation of I suspended in dilute hydrochloric acid8, is general and gives good yields of II as the hydrochloride salt. A typical procedure is given in the Experimental Section and several examples are listed in Table I.
Experimental
General Procedure
To a suspension of X g of an omega-nitrostyrene in X mL of conc. hydrochloric acid is added ca. 0.2-0.3 X grams of 10% Pd-C catalyst and enough water to make the omega-nitrostyrene comprise 5-7% of the mixture. Reduction is carried out at 50-80°C under 500-1500 psi of hydrogen in an agitated autoclave. Removal of the catalyst, evaporation of the filtrate, and trituration of the residue with acetone normally affords the hydrochloride salt of the β-phenethylamine pure enough for further use. In rare cases, when the hydrogenation filtrate is not colorless because of impurities in the omega-nitrostyrene, the aqueous filtrate is extracted with an immiscible organic solvent prior to evaporation.
Table I
β-Phenethylamine
Hydrochlorides (II*HCl)
| R | mp | Yield |
| 3-OH | 133-5°C11 | 84%a |
| 3-MeO | 147-8°C12 | 80%a |
| 3-OH, 4-MeO | 154-6°C3,b | 76%b |
| 3-MeO, 4-OH | 212-4°C3 | 82%a |
| 2,5-(MeO)2 | 138-40°C6 | 55%a |
| 2,4,5-(MeO)3 | 186-7°C13 | 72%a |
| 3,4,5-(MeO)3 | 181-3°C2 | 83%c |
- Recrystallized
- Free base
- Not recrystallized
β-(3,4-Methylenedioxyphenyl)ethylamine Hydrochloride
A rocking autoclave was charged with 207g (1.07 mol) of 3,4-methylenedioxy-β-nitrostyrene9, 207 mL of conc. hydrochloric acid, 65g of 10% Pd/C and the total volume was adjusted to 3500 mL by the addition of water. The mixture was hydrogenated at 55°C and an initial pressure of 800 psi until there was no further uptake, about 4 h. After cooling, the catalyst was removed and the colorless filtrate was evaporated to dryness under reduced pressure. Benzene was added to the residue and the evaporation was repeated to remove traces of water. The white, solid, residual β-(3,4-methylenedioxyphenyl)-ethylamine hydrochloride, after trituration with three 250 mL portions of acetone and drying at 70°C in a vacuum oven, weighed 153g (71%); mp 212-214°C10.
The β-phenethylamine hydrochlorides listed in Table I were prepared by this procedure from the corresponding ω-nitrostyrenes.