Abstract
Electroreduction of benzyl chlorides in the presence of acid anhydrides brought about stereoselective formation of (E)-enol esters of the corresponding benzyl alkyl ketones, the initial acylated products, in good to moderate yields.
Electrochemical acylation has been known as an efficient and useful method for reductive C-acylation of some of organic compounds,1 although it does not generate any acyl anions but gives the products which would be obtained from the usual chemical reactions of acyl anions2. For example, it was found that electroreduction of benzyl chlorides and related compounds in the presence of acid chlorides resulted in the reductive C-acylation to give the corresponding benzyl alkyl ketones in moderate yields3.
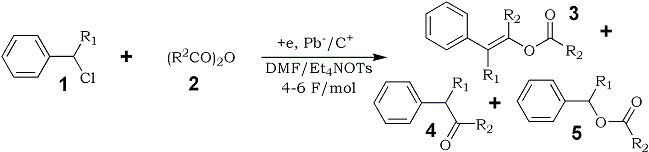
In this study, we report a unique electroreductive acylation of benzyl chlorides (1) with aliphatic acid anhydrides (2) to give the (E)-enol esters (3) of the corresponding benzyl alkyl ketones (4) as the main product in satisfactory yields. The corresponding benzyl alkyl ketones (4) and benzyl esters (5) were obtained as the by-products in small amounts.
Experimental
A typical procedure is as follows:
A solution of 20 g (0.067 mol) of tetraethylammmonium p-toluenesulfonate in 80 ml of anhydrous N,N-dimethylformamide was placed in cathodic (64 ml) and anodic (16 ml) chambers of a divided cell separated with a cylindrical ceramic diaphragm, to which a lead-plate cathode and a carbon-rod anode were attached. To a catholyte was added 2.53 g (0.02 mol) of benzyl chloride (1a) and 20.4 g (0.20 mol) of acetic anhydride (2a). The mixture was electrolyzed with stirring at room temperature under constant current condition (current density: 15-10 mA/cm2 ) until 4.0 F/mol of electricity has been charged. The usual work-up of the catholyte and the distillation of the product mixture gave 2.50 g of (E)-1-phenyl-2-acetoxypropene (3a) (yield: 71%), along with benzyl methyl ketone (4a) and benzyl acetate (5a) in 2% and 5% yields, respectively.
Note: By shaking the above enol ester (1-phenyl-2-acetoxypropene) vigorously with 5% NaOH solution, it is hydrolyzed quantitatively to P2P, making its total yield 73%.
This electroreductive acylation was influenced by current density and the kind of cathode material, and better results were obtained at current density of 15-10 mA/cm2 by employing a Pb or Sn plate for the cathode.
Table 1.
Acylation of Benzyl Chlorides with Acid Anhydridesa
Benzyl chlorides |
Anhydrides |
Products (%)b |
|||
X | R1 |
R2 |
3c |
4 |
5 |
H | H (1a) | CH3 (2a) |
71 (3a) | 2 (4a) | 5 (5a) |
H | H (1a) | C2H5 (2b) |
73 (3b) | 6 (4b) | 6 (5b) |
p-F | H (1b) | CH3 (2a) |
67 (3c) | 7 (4c) | 6 (5c) |
p-F | H (1b) | C2H5 (2b) |
72 (3d) | 9 (4d) | 6 (5d) |
p-Cl | H (1c) | CH3 (2a) |
56 (3e) | 7 (4e) | 8 (5e) |
p-Cl | H (1c) | C2H5 (2b) |
63 (3f) | 4 (4f) | 6 (5f) |
p-CH3 |
H (1d) | CH3 (2a) |
53 (3g) | 10 (4g) | 9 (5g) |
p-CH3 |
H (1d) | C2H5 (2b) |
53 (3h) | 18 (4h) | 11 (5h) |
p-OCH3 |
H (1e) | CH3 (2a) |
52 (3i) | 12 (4i) | 18 (5i) |
3,4-(CH3)2 |
H (1f) | C2H5 (2b) |
51 (3j) | 11 (4j) | 15 (5j) |
- Conditions: Pb cathode, current density 15 mA/cm2.
- Based on isolated products.
- The E/Z ratio of the stereoisomers was analyzed by
GLC technique (column: SE-30, 2 m) and was found
to be more than 99:1 for 3a-j.
A variety of substituted benzyl chlorides (1a-f) were electrochemically reduced in the presence of large excess of acetic or propionic anhydride (2a,b) under similar conditions as above. The corresponding (E)-enol esters 3a-j were formed stereoselectively as the main product in satisfactory yields accompanying with formation of benzyl ketones 4a-j and esters 5a-j as the by-products in small quantities, as shown in Table 1.4
(E)-Stereochemistry of 3a-j was determined by analysis of chemical shift of the vinyl protons (δ = 6.10-6.16 ppm (CDCl3)) in their 1H-NMR spectra. The vinyl protons of their (Z)-isomers are thought to appear in higher field by 0.2-0.4 ppm than those of the (E)-isomers since those of the (E)-isomer 3a and the (Z)-isomer of 1-phenyl-2-acetoxypropene appear at δ = 6.15 and 5.80 ppm, respectively.5
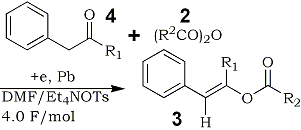
Initial formation of 4a-j and their subsequent transformation to 3a-j in this reaction were confirmed by controlled experiments.6 Thus, the electrolysis of 4a,b with 2a,b under the similar conditions, which gave 3a,b in almost quantitative yields, as shown in Table 2.
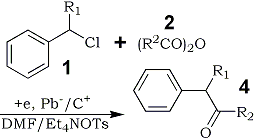
It may be noteworthy that the presence of an alkyl group at the α-position of the starting benzyl chlorides 1 in this electroreductive acylation brought about selective formation of ketones 4 (Table 3).
Table 2.
Electroreductive Acylation of Benzyl Alkyl
Ketones 4 with Acid Anhydrides 2
Ketone | Anhydride |
Product |
R1 |
R2 |
3 |
CH3 (4a) | CH3 (2a) |
91% (3a) |
CH3 (4a) | C2H5 (2b) |
94% (3k) |
C2H5 (4b) |
CH3 (2a) |
90% (3l) |
C2H5 (4b) |
C2H5 (2b) |
95% (3b) |
Table 3.
Electroreductive Acylation of α-Alkyl
Benzyl Chlorides 1 with Anhydrides 2
Chlorides | Anhydrides |
Products |
1, R1 |
2, R2 |
4 |
CH3 |
CH3 |
81% |
CH3 |
C2H5 |
88% |
C2H5 |
CH3 |
70% |
C2H5 |
C2H5 |
75% |
C3H7 |
CH3 |
71% |
C3H7 |
C2H5 |
79% |
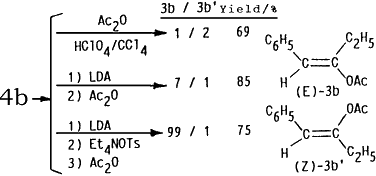
In order to explain high stereoselectivity on formation of (E)-enol esters 4 in this electrochemical acylation, conventional methods for preparation of enol acetates from ketones have been examined. An acid-catalyzed O-acylation (HClO4/Ac2O/CCl4)7 of benzyl ethyl ketone (4b) gave a stereoisomeric mixture of (E) and (Z)-1-phenyl-2-acetoxy-1-propene (3b and 3b') in the ratio of 1:2. On the other hand, treatments) of 4b with lithium diisopropyl amide (LDA) at -78°C followed by acetylation with Ac2O led to formation of 3b and 3b' in the ratio of 7:1 while the (E)-enol acetate 3b was exclusively obtained ((E)-3b/(Z)-3b' = 99:1) by addition of Et4NOTs after treatment of 4b with LDA and subsequent acetylation with Ac2O. Furthermore, reduction potential of 1a was -1.97 V (Ep vs. SCE) and 2a showed no reduction peak up to -2.50 V. This fact may indicate that the products 4 were initially formed by electron transfer to 1 followed by electrophilic attack of 2 in the present electroreductive acylation.
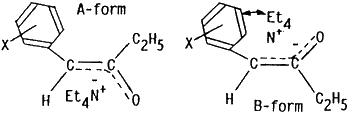
Therefore, coordination of sterically bulky tetraethylammonium cations (Et4N+) with negative charges on the oxygen and/or the benzylic carbon atoms of the anion species, possibly formed by direct electron transfer to the ketones 4 or their reactions with an electrogenerated base,8 may also make A-forms more favorably than B-forms because of steric hindrance between Et4N+ and phenyl rings in B-forms.