Organic Syntheses, Coll. Vol. 3, p.375 (1955); Vol. 28, p.58 (1948).
Submitted by Norman Rabjohn
Checked by H. J. Sampson and R. S. Schreiber.
1. Procedure
A report has been received that a sample of
ethyl azodicarboxylate
decomposed upon attempted distillation with sufficient violence to
shatter the distillation apparatus.
It is possible that the explosion may have been due to over
chlorination or to insufficient washing of the product with sodium
bicarbonate solution.
It is recommended that
ethyl azodicarboxylate be distilled only behind a safety shield, and protected from direct sources of light.
A.
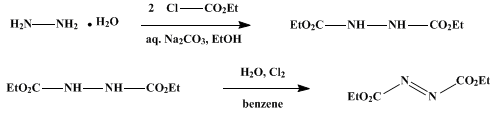
Ethyl hydrazodicarboxylate. In a
2-l. three-necked flask, equipped with a
mechanical stirrer,
two 500-ml. dropping funnels, and a
thermometer (Note 1), is placed a solution of
59 g. (1 mole) of 85% hydrazine hydrate in 500 ml. of 95% ethanol. The reaction flask is cooled by means of an
ice bath. When the temperature of the solution has dropped to 10°,
217 g. (2 moles) of ethyl chloroformate is added dropwise with stirring at a rate sufficient to maintain the temperature between 15° and 20°. After one-half of the
ethyl chloroformate has been introduced, a solution of
106 g. (1 mole) of sodium carbonate in 500 ml. of water is added dropwise simultaneously with the remaining
ethyl chloroformate.
The addition of these two reactants is regulated so that the
temperature does not rise above 20° and so that the addition of the
chloroformate is completed slightly in advance of the
sodium carbonate in order to ensure a slight excess of
ethyl chloroformate in the reaction mixture at all times.
After all the reactants have been added, the
precipitate on the upper walls of the flask is washed down with 200 ml.
of water and the reaction mixture is allowed to stir for an additional
30-minute period. The precipitate is then collected on a
Büchner funnel, washed well with a total of 800 ml. of water, and dried in an
oven at 80°. There is obtained
145–150 g. (
82–85%) of
ethyl hydrazodicarboxylate which melts at
131–133°. It is sufficiently pure
(Note 2) for the preparation of
ethyl azodicarboxylate.
B.
Ethyl azodicarboxylate. A mixture of
100 g. (0.57 mole) of ethyl hydrazodicarboxylate,
500 ml. of benzene, and 500 ml. of water is placed in a 2-l. three-necked flask equipped with a mechanical stirrer and a
gas inlet tube. The flask and contents are tared, the flask is placed in an ice bath, and a slow stream of
chlorine is bubbled into the mixture with stirring. The temperature is maintained below 15°, and
chlorine is introduced until the increase in weight amounts to 50–55 g.
(Note 3). The flow of
chlorine is stopped, and the reaction mixture is stirred until a clear, orange-colored
benzene layer forms when the mixture is allowed to settle.
The layers are separated, and the water layer is extracted once with
benzene. The
benzene solutions are combined and washed twice with 100-ml. portions of water, then with
100-ml. portions of 10% sodium bicarbonate solution until neutral (usually four to six washes are required), and twice more with water, and then are dried over anhydrous
sodium sulfate. The
benzene is removed under reduced pressure on a
steam bath, and the residue is distilled in vacuum through a
short indented column. After a small fore-run, the main fraction is collected at
107–111° /15 mm. There is obtained
80–82 g. (
81–83%) of
ethyl azodicarboxylate.
2. Notes
1.
The thermometer and one of the funnels are fitted to a
two-necked adapter;
the thermometer scale must be such that the range between 10° and 20°
is easily visible, preferably outside the flask, when the bulb is
inserted in the liquid.
3. Discussion
This preparation is referenced from:
Copyright © 1921-2007, Organic Syntheses, Inc. All Rights Reserved