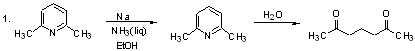
Catalytic hydrogenation pp. 219-232
Metal Hydride reductions pp. 232-253
Dissolving Metal reductions pp. 253-265
Misc. reductions
1. Catalytic Hydrogenation
Involves adding the elements of H2 across a double bond.
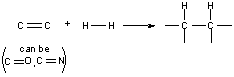
This reaction normally has a very high Ea. We can lower this Ea by use of a catalyst.
Heterogeneous reactions - Conditions are important:
- temperature
- pressure
- solvent
- catalyst (poisons)
Three classes of hydrogenation:
low pressure 1 atm (~14 psi)
medium pressure 14 - 100 psi
high pressure 1500 - 4500 psi
Catalysts:
Noble metals
Pt - from reduction of PtO2 in hydrog. apparatus.
Palladium
Ruthenium
Rhodium
(Ra-Ni - will say more about this later.)
Deposited on the surface of an inert support
(carbon, alumina, BaSO4, CaCO3)
---------------------------------------------->
catalyst activity decreases
Available from:
Johnson Matthey (Alfa)
Engelhard Industries, Inc.
Solvents
EtOH
MeOH
EtOAc
H2O
Cyclohexane
Dioxane
THF
Acetic acid
Catalyst activity increases on going to polar, acidic solvents.
Temperature: room temperature => 300°C
High pressure catalysts:
Ra-Ni - commercially available as W-2.
Higher grades (W-3 --> W-7) can be prepared.
Copper chromite CuCr2O4
Ruthenium supported on carbon or alumina.
Examples of some reductions:
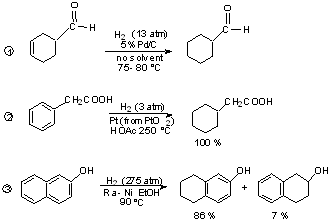
Scheme 5.3. Conditions for Catalytic Hydrogenation.
| Functional Groups | Reduction Product | Common Catalysts | Typical rxn conditions |
|---|---|---|---|
| C=C | H-C-C-H | Pd, Pt, Ni, Ru, Rh | Rapid at R.T. and 1 atm except for highly substituted or hindered cases |
| -C=C- | H-C=C-H | Pd | R.T. and low pressure quinoline or lead added to deactivate catalyst |
| Benzene | Cyclohexane | Rh, Pt | Moderate pressure (5-10 atm), 50-100°C |
| C6H5(CO)R C6H5CH(OR)R | C6H5CH2R | Pd | R.T., 1-4 atm, acid catalyzed |
| C6H5CH(NR2)R | C6H5CH2R | Pd, Ni | 50-100°C, 1-4 atm |
| R(CO)Cl | R(CO)H | Pd | R.T., 1 atm, quinoline or other catalyst moderator used |
| R-CN | RCH2NH2 | Ni, Rh | 50-100°C, usually high pressure, NH3 added to increase yield of primary amine |
| R(CO)NH2 | RCH2NH2 | Cu-Cr | Very strenuous conditions required |
| RNO2 | RNH2 | Pd, Ni, Pt | R.T., 1-4 atm |
| RC(=NR)R | R2CHNHR | Pd, Pt | R.T., 4-100 atm |
| R-Cl R-Br R-I | R-H | Pd | Order of reactivity: I > Br > Cl > F bases promote reaction for R = alkyl |
| Epoxides | ROH | Pt, Pd | Proceeds slowly at R.T., 1-4 atm, acid-catalyzed |
Hydrogenolysis - cleavage of a single bond by catalytic hydrogenation.
Allylic C-O or C-N
Benzylic bonds
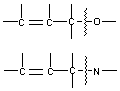
C- halogen
C-S
N-N
N-O
O-O
C-C
C-O 3-membered rings
C-N
Examples:
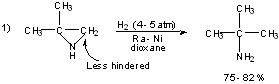
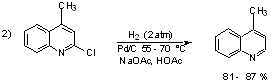
3) Rosemund Reduction
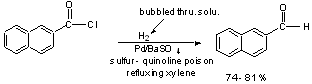
Typical Catalyst Poisons:
These can also be used to reduce the activity of the catalyst and make it more selective.
Mercury
Divalent sulfur compounds
Amines
Preferentially bond to the metal surface and thus prevent bonding between catalyst and molecule to be reduced.
Small amounts of impurities can sometimes be removed by stirring a soln. of starting material over Ra-Ni.
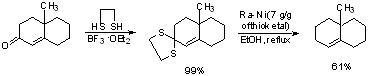
Raney-Nickel Desulfurization:
Mild method for reducing aldehydes/ketones to -CH2-.
Reductive Alkylation:
Conversion of a ketone to an amine.
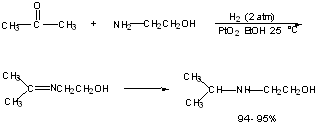
Also can use:
NaBH3CN
Sodium cyanoborohydride
Very specific for iminium groups -----> amines.
Mechanism and Stereochemistry:
In general, there is overall cis addition of H2 across C=C from less hindered side.
Example:
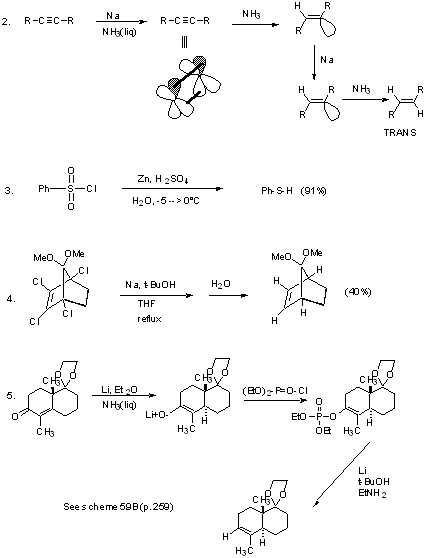
Disubstituted acetylenes -----> cis-olefins
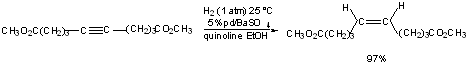
Also can use Lindlar's catalyst: Pd poisoned with Pb(OAc)2.
Works better for acetylenes than alkenes because they are more strongly adsorbed onto catalyst surface.
Why can't we deuterate C=C without scrambling?
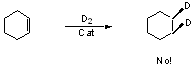
(but we can use Wilkinson's Catalyst - coming later)
From Scheme 5.1 (p. 221):
Syn addition to less hindered side.
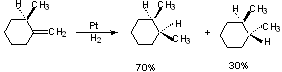
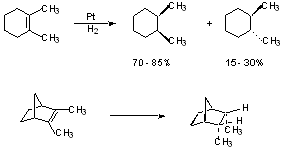
There are a number of exceptions, so you need to be careful.
Look at mechanism in detail:
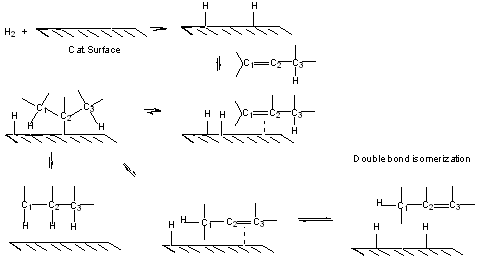
Hence olefins can be isomerized over hydrogenation catalysts.
Homogeneous Catalyst Hydrogenation:
Wilkinson's Catalyst: tris-(triphenylphosphine)rhodium chloride soluble in organic sovlents (EtOH, etc.)
Preparation and reaction:
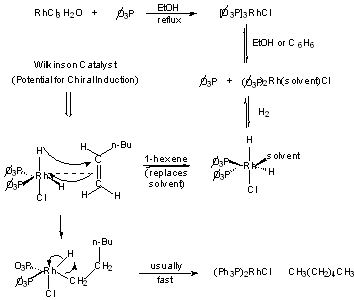
1. Useful for the stereospecific transfers of D2 to unhindered olefins:
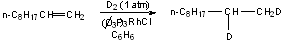
2. Reduces only unhindered C=C even in the presence of other labile groups (keto, nitrite, nitro, sulfide, etc.).
See Scheme 5.2 (p. 224)
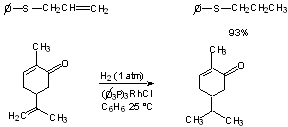
Enantioselective Hydrogenations
Chiral complexes with Ru and Rh lead to chiral induction in the hydrogenation process.
(See Table 5.1, p. 226-7)
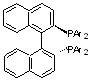
Dehydrogenation Reactions (actually included under oxidation)
Generally removal of hydrogen to from an aromatic system. The fewer the number of H's to be removed, the milder the conditions.
Reaction Conditions
Catalysts:
Sulfur
Selenium
Pd/C
Platinum
Several quinones
High-boiling solvents:
b.p. Cumene 153°C p-Cymene 176°C Decalin 185°C Quinoline 238°C Naphthalene 218°C Nitrobenzene 211°C
Reflux and stir.
Pass through stream of CO2 or N2 to sweep out the H2 which is given off.
Some restrictions on functional groups which can be present --
must be non-reducible
3° ROH dehydrate
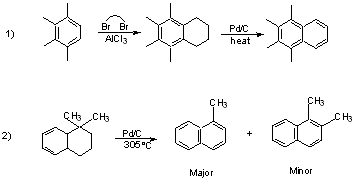
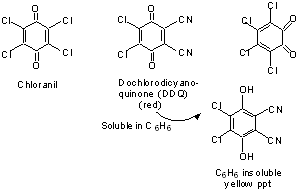
Quinones are widely used for dehydrogenation.
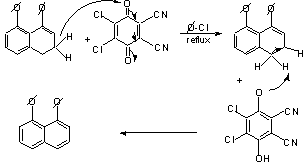
Let's look at the mechanism of dehydrogenation:
Section 5.2 - Group III Hydride Donors (A1 & B)
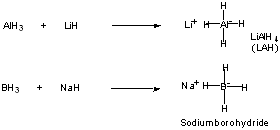
Table 5.2 (p. 232) Relative Reactivity
LiAlH4 most reactive Book shows different products from
NaBH4 less reactive C=O (type) reductions.
Also attack C=N, C=N, N=O, but not C=C.
Substituted Derivatives:
NaAlH2(OCH2CH2OCH3)2 Rd-Al
LiAlH2(OCH2CH2OCH3)2
LIAlH(OC(CH3)3)3 LiAlH4 + 3 C(CH3)3OH
NaBH3CN Sodium cyanoborohydride (only imines)
AlH3 Aluminum hydride
BH3 (B2H6) Diborane
[(CH3)2CHCH2]2AlH Diisobutylaluminum hydride (DlBAH)
[(CH3)2CHCHCH3]2BH Di-sec-amylborane (Disiamylborane)
9-Borobicyclononane (9-BBN)
Mechanism involves coordination of cation (Li+ or Na+) with C=O and H- attack.
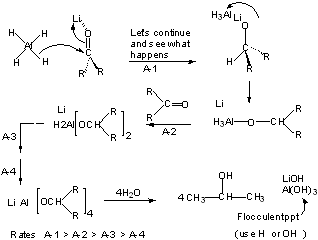
Thus substituted LiAlH4 are less reactive and hence more selective than LAH.
NaBH4 similar mechanism but different relative rates.
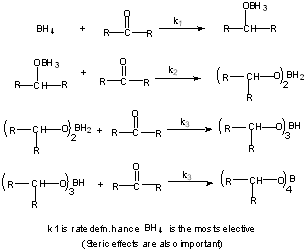
Reaction Conditions
Anhydrous conditions (except NaBH4)
LAH non-hydroxylic solvents: ether
THF
1,2-dimethoxyethane
(Me)-CH2CH2-O-CH2CH2-O-Me) diglyme
NaBH4 H2O, MeOH, EtOH
most commonly i-PrOH
Two principal factors must be controlled in the reduction of functional groups:
1. selectivity
2. stereochemistry
Selectivity
1. Partial reduction
i. e. RCOOH -----> RCHO
2. reduction of one group in the presence of another.
1. Partial Reduction
a) RCOOH ----> RCHO
easiest to go through RCOCl
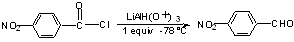
b) Lactones ----> Lactols
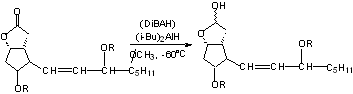
c) Partial Reduction of nitriles --> imines (can be hydrolyzed to aldehydes).
Normally with LAH
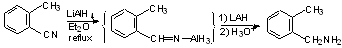
However, with hindered hydride, the 2nd step does not occur, and we can hydrolyze intermediate iminium salt to RCHO.
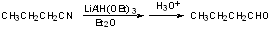
Can also use DIBAH to get even better yields
d) Reduction of amides
-----> amines
-----> aldehydes
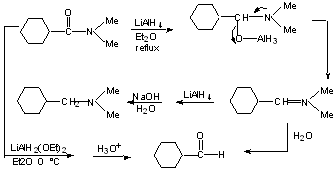
Also,
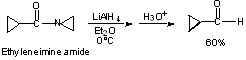
can't donate e- from N as well.
e) Enol Ethers of 1,3-dicarbonyl compounds.
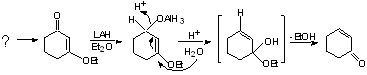
2. Reduction of One Group in Presence of Another
a) 1,2 vs. 1,4 Reduction of a,b-unsaturated carbonyl compounds.
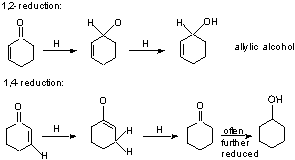
- LAH and NaBH4 give mostly sat'd alcohol (1,4)
- DlBAH and 9-BBN give exclusive 1,2 (C=O reduction)
- H2/cat often reduces only C=C
b) NaBH4 will reduce ketones in the presence of esters, amides, C=C, nitro, R-X.
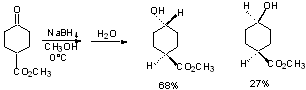
c) AlH3
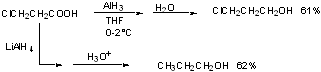
d) Tin Hydride will cleave some C-X bonds.
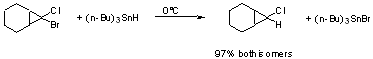
to prepare reagent:
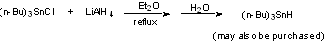
For other examples:
See Scheme 5.5 (p. 245) "Reduction of Other Functional Groups by Hydride Donors"
See Scheme 5.6 (p. 251) for other R3SnH reductions.
Stereochemistry of Ketone Reductions
Rule of Steric Control of Asymmetric Induction - Cram's Rule
Ketone will react preferentially in the conformation in which the carbonyl group is staggered between the medium and smallest substituents on the a-carbon.
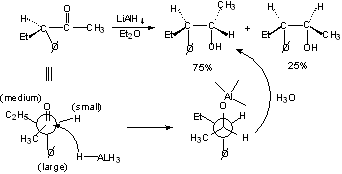
Carbonyl attacked from less hindered side.
Rigid Systems - cannot rotate as above. - attack occurs from less hindered side.
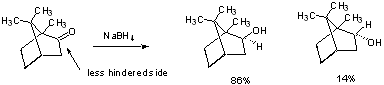
When steric environment on both sides of carbonyl is comparable, the more stable product is formed. But hindered hydrides show more selectivity.
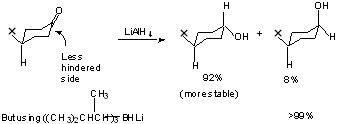
Table 5.3 Shows reduction of substituted cyclohexanone mediated by steric approach control.
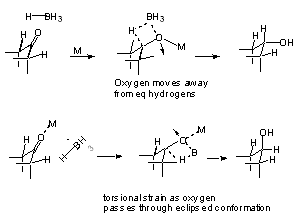
But if product development control does not function, then how do we explain the preference for equatorial alcohols where no over-riding steric factors exist??
Perhaps due to extra torsional strain in the transition state leading to axial alcohols.
Epoxide Reductions
LiAlH4 attack occurs at less hindered carbon:
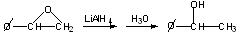
Cyclohexene oxides give trans-diaxial opening.
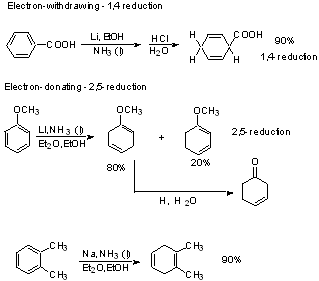
Dissolving Metal Reduction
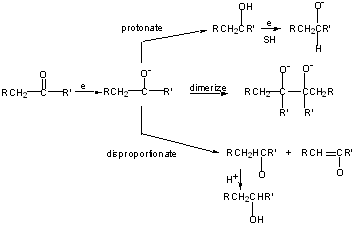
In general, we use this method to reduce conjugated systems, enones, or aromatics.
For saturated C=O compounds, hydride reductions are better.
Reaction Conditions
1) Metals commonly used:
lithium
sodium
potassium
(cesium)
(calcium)
zinc
magnesium
tin
iron
2) Solvents
NH3 (b.p. - 33˚) for alkali metals (and Calcium); "Birch Reduction"
low molecular wt. amines
hexamethylphosphoramide (HMPA)
ether, THF, dimethoxyethane (DME) -- dilute solns.
crown ether complexes
ether, toluene, xylene -- suspensions
3) Proton Source
ethanol -- Present in reaction medium
isopropanol -- added with compound to be reduced
t-BuOH -- added during isolation.
H20
4) Amalgams with Hg (Free metal and HgCl2)
Aluminum
Zinc
Tin
Stereochemistry
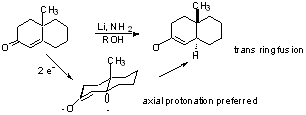
This preference may be precluded by the existence of other bulky groups wich affect the anion conformation.
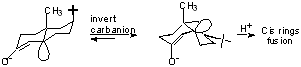
Sometimes possible to intercept radical anion before protonation (on oxygen).
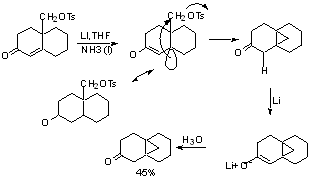
Further reduction can be accomplished in mixed amine solvents:
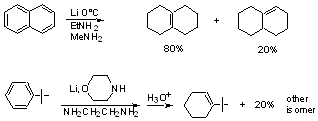
Effect of substituents
Electron donating substituents slow down rate
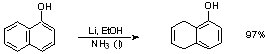
Orientation: See scheme 5.8 (p. 257)
Explain by intermediates such as
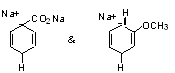
Reductive eliminations (from a-subst. C=O compounds)
Scheme 5.10 (p. 260)
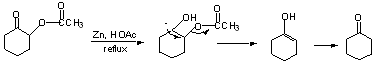
trans coplanar arrangement allows for maximum orbital overlap -- stereoelectronic control.
i.e.
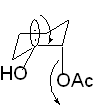
thus cyclohexanes with axial substitutents are cleaved more readily.
Dehalogenation
See Scheme 5.9 (p. 259) (Try to work some mechanisms).
2-haloketones
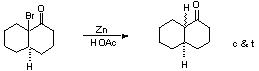
Clemmemsen Reduction
Other examples of C=O --> CH2
in Scheme 5.12 (p. 267)
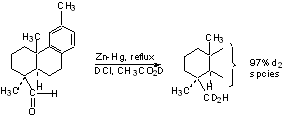
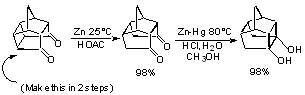
Reductive C-C Bond Formation
(a) Pinacol Formation
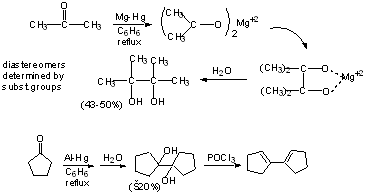
Aromatic Systems
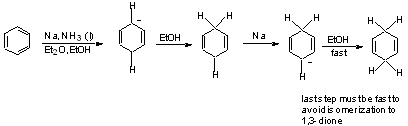
similarly:
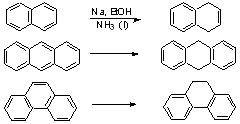
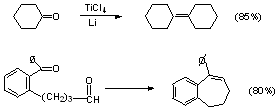
b) McMurry Reaction (see scheme 5.11 - p. 264)
Acyloin Cyclization
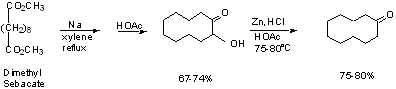
Work mechanism yourselves. (See p. 262)
Trap with Me3SiCl
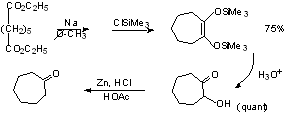
Keto-esters:
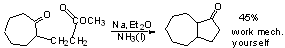
Reduction of Other Functional Groups