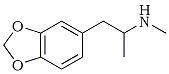
This article will present the factual material that exists in the literature representing the results of laboratory studies and scientific experimentation with methylenedioxymethamphetamine or MDMA, the common name applied to an organic compound, a secondary amine. As a free base it is a white, musty smelling oil with a searing taste, insoluble in water but soluble in most organic solvents. It forms salts with several acids, and these are white solids or oils that are readily water soluble and bitter to the taste. It has an empirical formula C11H15NO2, and its structural formula is given in Figure 1.
 |
MDMA | |
Family | MDMA Name | |
Structure | Name | |
Propane | 2-Methylamino- 1-(3,4-methylenedioxyphenyl)-propane | |
Isopropylamine | N-Methyl- β-(3,4-methylenedioxyphenyl)-isopropylamine | |
Ethylamine | N,α-Dimethyl- β-(3,4-methylenedioxyphenyl)-ethylamine | |
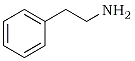 | Phenethylamine | N,α-Dimethyl-3,4-methylenedioxyphenethylamine |
Benzeneethanamine | N,α-Dimethyl- 3,4-methylenedioxybenzeneethanamine | |
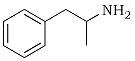 | Amphetamine | N-Methyl-3,4-methylenedioxy-amphetamine |
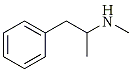 | Methamphetamine | 3,4-Methylenedioxy-methamphetamine |
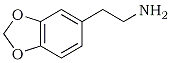 | Homopiperonylamine | N,α-Dimethylhomopiperonylamine |
Benzodioxole- 5-ethanamine | N,α-Dimethylbenzodioxole-5-ethanamine | |
MDMA has a number of correct chemical names, each based on one portion or another of the chemical structure. With that defining portion named as a stem word, the full chemical name is apparent by the additions to this base fragment. In Figure 1, the fragments (with their names) are drawn on the left and the extended name that applies to MDMA is given on the right. The use of the simplest aliphatic chain (i.e., ethylamine or isopropylamine) occurs in part to avoid the generic name amphetamine or methamphetamine. These terms are so frequently used that each listener conjures up an image of the chemicals being described, according to his/her discipline: the chemist sees the carbon chain, the pharmacologist sees the stimulant, and the policeman sees the drug laws.
| S-MDMA (+) | 66142-89-0 |
| S-MDMA (+) HCl | 69558-32-3 |
| R-MDMA (—) | 81262-70-6 |
| R-MDMA (—) HCl | 69558-31-2 |
| MDMA (racemic) | 69610-10-2 |
| MDMA HCl (racemic) | 64057-70-1 |
The names that are to be used in the searching of Chemical Abstracts depend on the date of the search. ln the earliest files, the homopiperonylamine name was used, and then up to 1972, MDMA was entered with the phenethylamine name. Since then, the heterocyclic term benzodioxole-5-ethanamine has been the root name. The common abbreviation MDMA is based on the consideration of the structure as a substituted methamphetamine. Other terms that have been used to refer to this drug include MDM, Ecstasy, XTC, Adam, and EA-1475 (the last, by the Edgewood Arsenal). The computer searching of Chemical Abstracts employs the following registry numbers:
Chemistry
Synthesis
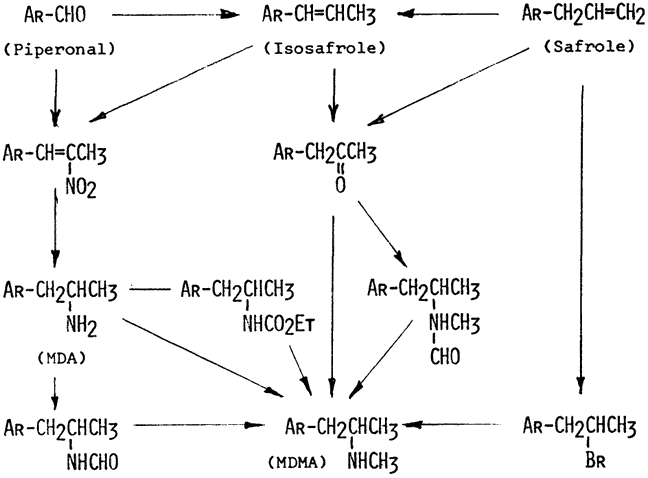
There are six methods of preparation to be found in the scientific literature. ln all cases, the starting material carries the preformed methylenedioxy ring, in the form of safrole, isosafrole, or of the derived aldehyde, piperonal. The first preparation and description of MDMA was a German patent issued to the firm E. Merck (1914) in Darmstadt, dated December 24, 1912, and made available May 16, 1914. Here, MDMA was synthesized in two steps from safrole. The addition of aqueous hydrobromic acid provides an impure intermediate (1-methylenedioxyphenyl-2-bromopropane) that is converted with an alcoholic solution of methylamine to MDMA. The same process, except for the isolation and purification of the bromo intermediate, was described by Polish chemists almost 50 years later (Biniecki & Krajewski 1960).
MDMA has also been synthesized from MDA by reaction with ethyl chloroformate, followed by reduction with Red-Al (Davis & Borne 1984). Similarly, MDA can be converted to the formamide that is reduced with lithium aluminum hydride in tetrahydrofuran (Braun, Shulgin & Braun 1980a). One report (O'Brien, Bonicamp & Jones 1982) described the methylation of MDA with methyl iodide. MDMA was obtained, but the dimethylated tertiary amine and the trimethylated quaternary products were also generated as contaminants.
Two procedures exist for the synthesis of MDMA by the reductive amination of piperonylacetone with methylamine. The reducing agents are either sodium cyanoborohydride in methanol (Braun, Shulgin & Braun 1980a) or amalgamated aluminum in aqueous isopropanol (see Nichols, in Frith 1986b). The cyanoborohydride method has been used for the preparation of tritium-labeled MDMA using labeled methylamine (Gehlert et al. 1985). Piperonylacetone may also be reacted with N-methylformamide in the Leuckart reaction, and MDMA obtained by the hydrolysis of the intermediate N-formyl derivative (Bailey et al. 1975). This N-formyl intermediate is also the topic of an early German patent describing its formation from MDMA and chloral hydrate (Merck 1920).
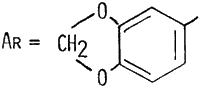
The piperonylacetone required for these syntheses is available commercially. (See comments below for labeling misidentifications.) It can also be made either by the reduction of the nitroethane adduct of piperonal with elemental iron or the oxidation of isosafrole with hydrogen peroxide in formic acid.
Figure 2.
Synthetic routes to MDMA
"Piperonyl-" |
Piperonylacetone |
|
 | Ar-CH2COCH3 | (the "right" ketone) |
Ar-CH2CH2COCH3 | (the "wrong" ketone) | |
Synthetic Precautions
Some potential synthetic mishaps should be considered. Substitution of isosafrole for safrole leads, in the reaction with hydrogen bromide, to an isomeric bromopropane intermediate that on amination with ammonia produces an α-aminated analogue of MDMA (Merck 1914). Presumably, the substitution of the methylamine, as in the procedure above, would produce 1-(3,4-methylenedioxyphenyl)-1-methylaminopropane, the benzylamine isomer of MDMA.
In the syntheses starting with piperonylacetone, the substitution of 1-(3,4-methylenedioxyphenyl)-3-butanone for 1-(3,4-methylenedioxyphenyl)-2-propane (an error that as been made by commercial suppliers of piperonylacetone) leads to the formation of 1-(3,4-methylenedixoxyphenyl)-3-methylaminobutane (HMDMA); with ammonia rather than methylamine, this incorrect starting material would lead to 1-(3,4-methylenedioxyphenyl)-3-aminobutane (HMDA) (Shulgin & Jacob 1982a, 1982b). The structure for this alternate "piperonylacetone" is also given in Figure 2.
Only a modest pharmacological literature exists on these two aminated homologues. One study (Kasuya 1958) has compared HMDMA with atropine and found it to be a weak spasmolytic. The toxicity and pharmacology of this homologue in mice (and of the corresponding MDA homologue HMDA) have been studied and published (Davis & Borne 1984). The primary amine HMDA was found to be inactive (in rats at 10 mg/kg) in both open field testing and as a stimulant (Buxton 1972), but at higher doses caused slight stimulation with tremors, and modest inhibition of monoamine oxidase (Fellows & Bernheim 1950).
Physical Properties of MDMA
The free base has a boiling point in vacuo of 155°C/20 mmHg (Merck 1914) and 110-120°C/0.4 mmHg (Braun, Shulgin & Braun 1980a). The hydrochloride salt can occur in a number of hydrated crystalline forms, making the physical properties and solid spectra of risky value for identification and as criteria of purity. The following melting points (mp) are given: for anhydrous, 147-148°C (Bailey et at. 1975), 148-149°C (Biniecki & Krajewski 1960), 148-150°C (Davis & Borne 1984; Merck 1914), 150-151°C (Gaston & Rasmussen 1972), 151-152°C (Braun, Shulgin & Braun 1980a), 152-153°C (Braun, Shulgin & Braun 1980a), 158-159°C (Nichols, In: Frith 1968b); for quarter-hydrate, soften 132°C and mp 135-139°C (Shulgin 1986); for hemihydrate, soften 92°C and mp 138-145°C (Shulgin 1986); for three-quarter hydrate, soften 50°C and mp 90-132°C (Shulgin 1986); for monohydrate, soften 80°C and mp 107-133°C (Shulgin 1986).
It is apparent that with uncertain hydration, the melting point is not an acceptable criterion of identity or of purity. Each of these polymorphs has, however, a distinct and characteristic crystalline polymorphic structure. The index of refraction has been determined: n19D = 1.5311 (Biniecki & Krajewski 1960).
A considerable body of spectral data exists for MDMA. As mentioned above, the several polymorphs of the hydrated hydrochloride salts have distinct infrared spectra. Some of these are shown in Figure 3. The spectra of the free base (Nichols, In: Frith 1986b; Bailey et al. 1975) and the anhydrous hydrochloride salt (Bailey et al. 1975; Gaston & Rasmussen 1972) have been published. The latter are as KBr pellets, a spectral procedure that can dehydrate a material during preparation.
Figure 3.
MDMA IR spectra
Figure 4.
MDMA MS spectrum
The ultraviolet spectrum is characteristic of the methylenedioxyphenyl ring (as the hydrochloride in ethanol, 286 nm, e = 3843 [Bailey et al. 1975]; as the sulfate in water, 0.1 N, 284 nm, A 1% 1 cm = 164 [Gaston & Rasmussen 1972]). It is excellent for quantitative analysis, but is of little value for qualitative identification. The nuclear magnetic resonance spectra have been published, in part, both as the free base in CDCl3 and as the HCl salt in D2O by Bailey and colleagues (1975) and, in full, by Nichols (In: Frith 1986b). Mass spectral data have also been published (see Figure 4), both with electron impact (Bailey et al. 1975) and with chemical ionization (Nichols, In: Frith 1986b).
Analytical Procedures
Chromatographic analytical schemes have been developed. Two thin-layer chromatographic reports have appeared, one with six solvent systems (Bailey et al. 1975) and another with two, but with a progressive color development technique (O'Brien, Bonicamp & Jones 1982). A third study (Shaw & Peel 1975) was erroneously titled MDMA and actually investigated MMDA. Several reports of gas chromatographic analyses have been published, and this technique appears to be an excellent measure of both identity and purity (Nichols, In: Frith 1986b; Gupta & Lundberg 1977; Bailey et at. 1975; Gaston & Rasmussen 1972).
Toxicology
The mean lethal dose (LD50) of MDMA has been determined in several animal species. The first thorough study of the toxicity and behavioral pharmacology of MDMA was conducted at the University of Michigan during the period 1953-54, and was supported by a contract from the Army Chemical Center. The results were declassified in 1969 and published four years later (Hardman, Haavik & Seevers 1973). In this study, a total number of eight drugs were studied in five animal species. In all five species examined in this study, MDMA proved to be less toxic than MDA, but more toxic than mescaline. A number of other studies, often to determine behavioral responses or sublethal morbidity, have provided additional data. These are presented here by animal species.
- Mice
- The seminal study of Hardman, Haavik and Seevers (1973) determined the LD50 of MDMA in mice to be 97 mg/kg following intraperitoneal (i.p.) administration. More recent studies by Davis and Borne (1984) provided the same value (98 mg/kg i.p.) in isolated test animals. Aggregate toxicity, however, was found to be considerably higher (20 mg/kg), with a number of deaths being delayed. This latter value was also reported in conjunction with locomotor activities (Harris 1985).
- Rats
- The study by Hardman, Haavik and Seevers (1973) reported the LD50 in rats to be 49 mg/kg i.p. Orally, however, MDMA is less toxic, with an LD50 in rats of 325 mg/kg being reported (Goad 1985).
- Guinea Pigs
- The study by Hardman, Haavik and Seevers (1973) reported an LD50 in guinea pigs of 98 mg/kg i.p.
- Dogs
- In dogs, following intravenous injection, the LD50 was reported to be 14 mg/kg (Hardman, Haavik & Seevers 1973). The death of one dog was observed at an oral dose of 18 mg/kg in toxicity trials preliminary to behavioral studies (Frith 1986a). In this latter study, however, chronic oral treatment of 15 mg/kg led to no further deaths.
- Monkeys
- Intravenous administration of MDMA to monkeys (Macaca mulatta) provided an LD50 of 22 mg/kg (Hardman, Haavik & Seevers 1973).
Several studies have been made of toxicological changes in chemistry or body condition of both rats and dogs at sublethal levels of MDMA. Studies in subacutely treated rats (subcutaneous administrations twice daily for four days at 10, 20 and 40 mg/kg) led to extensive decrease of hippocampal serotonin levels as seen in post-mortem assays after a two-week wait. There was little change in either norepinephrine or dopamine levels (Woolverton et al. 1985). A single injection at the highest level produced a similar depletion (76% rather than 88%, relative to control animals). A preliminary report of these findings was submitted as evidence to the Drug Enforcement Administration (DEA) hearings on MDMA (Seiden 1985), and it was a report of parallel findings in the rat following MDA administration (Ricaurte et al. 1985) that was used to support the emergency scheduling of MDMA. Similar findings were reported, as a preprint to the DEA for use at the MDMA hearings, by Schmidt, Wu and Lovenberg (1985) and later published as an abstract (Schmidt & Lovenberg 1986). They too found that administration of high acute dosages of MDMA in rats depleted brain serotonin. They also found that pretreatment of the test animal with an antidepressant (citalopram) known to block serotonin uptake mechanisms prevented this decrease in serotonin. These findings are in agreement with studies of the levels of brain enzymes that are involved with the formation of neurotransmitters (Stone et at. 1986). Tryptophan hydroxylase activity in rats treated with MDA or MDMA (10 mg/kg subacutely) decreased in certain brain areas, unlike the decrease in tyrosine hydroxylase associated with high-level administration of methamphetamine. Rats administered MDMA (or separately, MDA) subacutely (10 mg/kg subcutaneously) were shown to have an increased neurotensinlike immunoreactivity level in certain regions of the brain (Merchant et at. 1986).
In a separate study (Goad 1985) of subacutely treated rats (oral administrations daily in increasing increments of 25 mg/kg per day for 13 days), survivors were sacrificed for tissue and brain pathology studies after a three-week wait. There were blood indicators of damage to both liver and kidney, but histological studies of brain tissue revealed no pathology (Frith 1986b).
Dogs administered MDMA on a chronic basis at oral dosages of up to 15 mg/kg/day showed restricted weight gain, and in several males at the highest dosages, testicular atrophy. Observed possible central nervous system (CNS) lesions were believed to be artifacts (Frith 1986a).
Pharmacology
In vitro Studies
Studies have been conducted using various in vitro systems for the purpose of evaluating the relationship between MDMA and various neurotransmitters. Most frequently, the neurotransmitter serotonin has been the focal point of these studies. Assaying the optical isomers of MDMA (in rat brain synaptosomes), Nichols and colleagues (1982) have found that the enantiomer of MDMA effective in humans (the S or + isomer) is the more effective isomer in releasing serotonin. The study of the optical isomers of MDMA on the inhibition of the uptake not only of serotonin, but of other neurotransmitters, is the subject of a recently completed master of science thesis (Steele 1986), which has been publicly presented (Steele, Nichols & Yim 1986). Studies have been made to determine the affinity of both MDA and MDMA for serotonin and dopamine receptors (Lyon, Glennon & Titeler 1986). Tritiated serotonin and ketanserin were used to label 5-HT1 and 5-HT2 receptors respectively, and the dopamine receptors were labeled with N-methylspiperone. All studies indicated a moderate affinity for the 5-HT2 receptors, with less for the 5-HT1 and very much less for the dopamine receptors. ln all cases the R isomer was more effective than the S isomer, with the racemate being intermediate in effectiveness. As the S isomer of MDMA is the more effective in humans, it was felt that these findings indicate a possible amphetamine-associated mechanism, rather than just serotonin involvement. Specific binding of radio-labeled MDMA in rat brain homogenates has been reported (Gehlert et al. 1985), and several drugs were evaluated as inhibitors of binding or as displacing agents. Studies observing neurotransmitter release in rat brain striatal slices showed MDMA to have a potency similar to the neurotoxin para-chloroamphetamine in the release of serotonin. Dopamine was found to be less affected (Levin, Schmidt & Lovenberg 1986). In general, these studies tend to imply some functional role of serotonin in the mechanism of action of MDMA.
In vivo Studies
Studies have been conducted on both restrained (electrodes, thermocouples) and freely moving animals (drug discrimination, behavioral pharmacology). A single report involved brain biochemistry with indwelling electrodes (Takeda et al. 1986) measuring MDMA-induced efflux of neurotransmitters by voltammetry in anesthetized rats. It was felt that the small amount of dopamine release seen might be due to the changes seen in serotonin levels.
An experimental procedure has been developed that shows a remarkably good correlation between the qualitative nature of a drug-induced rise in a rabbit's temperature (measured rectally) and the stimulant or psychotomimetic character of the tested drug. The extent of this temperature rise is proportional to the potency of the tested drug as a psychoactive agent in humans (Aldous et al. 1974). This assay, when applied in rabbits to the optical isomers of MDA and MDMA, showed a reversal of potencies of the isomers (Anderson et al. 1978). Thus, with MDA the R (levo-, l-) isomer is more potent than either the S isomer or the racemate, whereas the S (dextro-, d-) isomer of MDMA is the more potent. This is true in rabbit studies and in human evaluations as well. This reversal of active isomer assignment, coupled with the absence of cross-tolerance between MDMA and MDA in humans, supports the hypothesis that these two drugs have different mechanisms of action.
Drug Discrimination
A pharmacological technique that recently has been quite popular as a tool for comparing psychoactive drugs in experimental animals is the drug-discrimination assay. ln this assay, test animals are trained to discriminate between a given active compound and (usually) saline. Then the behavior seen resulting from varying dosages of a trial drug allows some qualitative assignment of action. Furthermore, two experimental drugs may be compared and against the other in order to determine their relative quantitative ranking.
Studies with rats trained to discriminate between d-amphetamine and saline or, separately, between MDA and saline, have shown MDMA in both cases to generalize to the drug in preference to the saline (Glennon & Young 1984). This, together with the findings that MDMA did not (unlike MDA) generalize to animals trained to discriminate 2,5-dimethoxy-4-methyl-amphetamine (DOM) from saline (Glennon et al. 1982), suggested that N-methylation of MDA (to produce MDMA) emphasizes the stimulant properties in preference to the psychedelic properties. In separate studies, however, rats trained to discriminate between d-amphetamine and saline, MDMA was found to only partially mimic d-amphetamine (Woolverton et al. 1985). In rhesus monkeys trained to discriminate between d-amphetamine and saline, MDMA appeared to be amphetaminelike, whereas MDA showed only partial mimicking of d-amphetamine (Woolverton et al. 1985).
Behavioral Pharmacology
A number of studies were made of MDMA, in comparison to both the primary amine (MDA) and the higher N-homologues, both as an analgesic and as a CNS stimulant in mice (Braun, Shulgin & Braun 1980a, 1980b). MDMA proved to be the most effective analgesic of all compounds tested, especially in the test that measures the loss of stretch reflex as a response to injected acetic acid. MDMA, and the immediate N-ethyl homologue MDE, were the most effective compounds in promoting motor activity. In this assay, they had more than twice the potency of MDA as stimulants.
Many of the observations on drug-induced behavioral changes are natural consequences of toxicity studies, and hence often reflect doses that approach, and in some cases exceed, the LD50 levels. When near-lethal amounts of MDMA are given to mice, the observed behavior has been described as being excitatory in nature (tremors, jerking, head clonus that progressed to clonic convulsions). Tonic seizures did not occur (Davis & Borne 1984). In discriminative stimulus studies conducted in rats (Glennon & Young 1984) doses in excess of 1.6-3.0 mg/kg could not be considered, due to behavior disruption (i.e., lack of any response at all). Hardman, Haavik and Seevers (1973) made behavioral observations of MDMA in the dog and in the monkey at substantially lethal doses (in the dog, between five and 50 mg/kg, with the LD50 = 14 mg/kg; in the monkey, between 10 and 75 mg/kg, with the LD50 = 22 mg/kg). Under these conditions, a spectrum of behavior similar to that of mescaline was observed (mescaline dose range in the dog, five to 60 mg/kg, with the LD50 = 54 mg/kg). This spectrum initially included motor effects (a weakness and a fluttering motion in the hind limbs) followed by salivation, emesis and defecation. A picture of disorientation and fear was presented for mescaline, and MDMA (in adequate doses) was said to parallel this picture, but no explicit details were given. These effects were apparently not seen in the monkey in this study. A similar study in the macaque (Schlemmer, Montrell & Davis 1986) at doses of up to 10 mg/kg showed some disruption of social behavior (i.e., self-grooming, food foraging), but no actions that suggested hallucinatory effects.
In rats, with orally administered MDMA, there were adverse clinical signs — largely related to excitability (i.e., piloerection, uncontrolled urination) — seen in all studies at or above 25 mg/kg. At higher levels (to 300 mg/kg) there were tremors and convulsions observed, with death resulting above this dose (Frith 1986b; Goad 1985). In similar studies with dogs administered near-lethal levels of MDMA orally, toxic behavioral signs were observed, such as rapid breathing, salivation and hyperactivity (Frith 1986a).
Two studies were solicited by the federal government to evaluate the abuse potential of MDMA through reinforcement studies (self-administration) in cocaine-trained primates. The first of these employed pretrained baboons (Griffiths, Lamb & Brady 1985) and found that two out of three animals reinforced themselves with MDMA, but with less intensity than with cocaine. The third animal did not self-administer MDMA on initial trials, but appeared to do so on retrial. A second study (Harris 1985) employed rhesus monkeys, also pretrained to self-administer cocaine. Again, some reinforcement was found in two out of three animals, suggesting a real abuse potential for MDMA.
Psychopharmacology
Nonclinical Studies
The earliest reports of human activity of MDMA were from research studies that were not clinically oriented. The first description of its action in humans (Shulgin & Nichols 1978) stated that it evoked an easily controlled altered state of consciousness, with emotional and sensual overtones. It shared a property with low levels of MDA in that it had little hallucinatory effect. A subsequent report (Shulgin 1983) elaborated more on the quality of action.
Most of the known psychedelic drugs suffer a major loss of potency on N-methylation (Anderson et al. 1978). MDMA is the one exception to this rule as it, like amphetamine, maintains potency as the N-methyl homologue. This pair is set apart also by the reversal of optical isomer configuration required for human activity, and the fact that there is no observed cross-tolerance between MDA and MDMA (Anderson et al. 1978).
From a large number of clinical trials, it became increasingly apparent that MDMA was without the harshness and complexity usually seen with MDA. This, coupled with the reversal of the optical isomer requirement for optimum human response, led to a firmer statement of the differences between these two drugs (Braun, Shulgin & Braun 1980a).
Clinical Studies
The most complete publication of the clinical application of MDMA in therapy appeared in 1983 (Greer 1983). It described the results of the administration of MDMA to 29 patients in a therapeutic setting. It concluded that the best use of MDMA is as an adjunct to insight-oriented psychotherapy to facilitate communication and intimacy between people involved in emotional relationships as well as in the treatment of alcohol and other drug abuse. It was emphasized that MDMA does not lend itself to overuse, because its most desirable effects diminish with frequency of use.
A study involving 13 experimental subjects was conducted in March 1985 (Greer 1985b) with the overseeing of an equal number of psychiatrists or psychotherapists, most of whom were experienced with both MDMA and LSD actions in humans. An extensive subjective analysis was made to develop a comparison between MDMA and LSD as potential therapeutic adjuncts. The principle effects of MDMA lasted three to five hours, while those of LSD are known to extend up to 14 hours. The clinicians agreed that MDMA was much easier to use than LSD, and because MDMA did not threaten ego control, involving little psychological risk to a naive subject. While LSD subjects sometimes experience transient delusional states, the only complications of using MDMA, according to the clinicians and researchers, are occasional anxiety and various physical symptoms due to the sympathomimetic effects of the drug. A description of the clinical protocol employed in MDMA therapy has been written and submitted as a chapter in a forthcoming book (Tolbert & Greer, in press).
A remarkable collection of anecdotal reports of MDMA use has recently appeared, describing more than 20 personal experiences. These first-hand accounts will be of keen interest to students of psychology (Adamson 1985).
The pharmacological and psychopharmacological findings related to MDMA have been summarized in several reviews (Nichols & Glennon 1984; Glennon, Rosencrans & Young 1983; Stafford 1983; Weil & Rosen 1983; Shulgin 1982, 1981, 1978). Most of these summaries were written by the authors of the original scientific studies and there are additional data included in these reviews.
Legal History
The initial proposal for the scheduling of MDMA appeared on July 27, 1984 (Mullen 1984a). Here was presented the usual body of justifications for the scheduling of an abused drug, and there was the pro forma request made for comments, with none expected. Comments were indeed made, however, and a second entry appeared on December 31, 1984, noting that hearings were to be held (Mullen 1984b). The date of February 1, 1985, was set as a time to hold a hearing to establish procedures, dates and locations. These hearings were held in 1985 in Los Angeles, Kansas City, Missouri, and Washington, D.C., and were presided over by an administrative law judge, Francis L. Young.
A request appeared in March 1985 for any and all information concerning illicit trafficking and medical problems associated with MDMA use (Unsigned 1985a). This was followed, at the end of May 1985, by a notice that appeared in the Federal Register (Lawn 1985) announcing the temporary placement of MDMA into Schedule I by the invocation of the emergency scheduling powers granted by the Comprehensive Crime Control Act of 1984. The effective date for this scheduling was July 1, 1985. This occurred in the middle of the hearings that were designed to determine the legal fate of MDMA as to its potential scheduling.
In an administrative development initially independent from the scheduling procedures initiated by the DEA in 1984, there was a request made through the Food and Drug Administration (Randolph 1984) for comments concerning the medical usefulness and abuse potential of some 28 drugs that were being considered by the World Health Organization for international restriction. MDMA was explicitly included on this list.
Popular Opinions
An unusually large amount of commentary and opinion has appeared in the popular press and in both professional as well as lay journals. Occasionally there may be some statements of fact, but usually there is much misstatement of fact.
The popular press has shown a blend of curiosity and sensationalism. There were sounds and shades of the LSD notoriety of the 1960's in that each reporter obtained some facts, but also borrowed details from other writers. The results were an oft-repeated story, generally moderately accurate and somewhat favorable. An issue of Brain-Mind Bulletin (April 15, 1985) was devoted to the controversy, and a short critical review appeared in the PharmChem Newsletter (Seymour 1985). In addition, the author of the present article has written a hypothetical question-and-answer interview (Shulgin 1985).
Articles or commentary also appeared in magazines and newspapers, such as Daily Californian (Marks 1986), High Times (Smith & Seymour 1986), New Age (Abramson 1985), Newsweek (Adler 1985), Chemical and Engineering News (Baum 1985), San Francisco Chronicle (Butler 1985), Oakland Tribune (Dentinger 1985), Life (Dowling 1985), San Francisco Examiner (Flinn 1985), Boston Globe (Foreman 1985), Alcoholism & Addiction (Gold 1985), Vanguard Press (Hudson 1985; Stevens 1985), Dallas Times Herald (Jubera 1985), New York Magazine (Klein 1985), Washington Post (Leavy 1985), Rolling Stone (O'Rourke 1985), Business Week (Schulman 1985), Psychology Today (Shafer 1985), Omni (Siegel 1985), Oklahoma Gazette (Siens 1985), Detroit News (Tessler 1985), Time (Toufexis 1985), Scientific American (Unsigned 1985b), San Francisco Bay Guardian (Wolfson 1985), The Rocket (Eichhorn 1984) and Substance Abuse Report (Unsigned 1984a). It even made the comics page, in Doonesbury (Trudeau 1985), and the editorials on KCBS Radio (Barnett 1985). Two long essays (Beck 1986; Ehrlich 1986) and a complete book (Seymour 1986) have appeared covering the subject.
One of the first promotional hypes for MDMA appeared in an underground magazine titled Wet (Unsigned 1981), in which the name Ecstasy was used and availability was implied as early as 1976. Another irresponsible tract appeared (Unsigned 1984b) that was styled to disarm and discourage the potential user of MDMA. This is an excellent example of inaccurate and misleading information where much detail that applies to MDA is ascribed to MDMA.
A perspective article (Riedlinger 1985) reviewed the recent history of MDMA and speculated on a number of areas of potential value. Grinspoon and Bakalar (1986a) presented an argument to the medical community supporting the need of drugs as adjuncts to psychotherapy, as well as having editorialized (Grinspoon & Bakalar 1986b) on the relationship between designer drugs and the law, using MDMA as an illustration. The broader question touching on the need of an acknowledgment of the value of consciousness alteration in society (using MDMA as a point of departure) has been presented to the lay community (Roberts 1986b). Several informational articles or tracts have appeared that seem reasonably neutral, but emphasize clinical utility nonetheless; they are apparently intended to simply provide information (Greer 1985a; Greer & Strassman 1985; Grinspoon & Bakalar 1985).
On the let's-discourage-drug-use-and-abuse side, there have been some noteworthy examples. A short review article in the American Psychological Association's APA Monitor (Turkington 1986) quotes statements (see below) ascribed to the authors of the rat serotonin studies. Another example is a newsletter on drug abuse (Cohen 1985) that equated all claims for MDMA to those that gave LSD and other psychedelics such glowing press years ago. It is stated that any attempts to set MDMA apart from MDA, DOM or PMA (or from the user-attestment record, from LSD or opium) reflects a lack of knowledge about these drugs. Furthermore, it indicated that MDMA appears to be less safe than LSD, and even LSD was a failure.
In addition, some organizations and federal agencies have produced tracts and flyers that are directed to the potential MDMA user, but have been written without much factual accuracy. Two examples are Do It Now Foundation's MDA/MDM (Dye 1982), and NIDA's "MDMA" (NIDA 1985), a government bulletin warning of potential psychotic episodes (wherein most information has been taken from the MDA record).
Conclusion
One of the inescapable facts of life is that with MDMA, as with everything that combines both promise and threat, there are intense protagonists and intense antagonists. And both groups are vocal.
From the promotional flyer (Dye 1982) mentioned above: "When people feel well, centered, unthreatened and aware of their own strength and loveliness, they are able to drop many of the usual barriers. Habitual users of tobacco have no need to smoke. Chain smokers of marijuana do not need their weed. Nail biters leave their fingers alone. Compulsive talkers become quiet," and on and on: pretty much a glowing picture, without negatives.
And the opposite extreme is just as unrelenting. From the APA Monitor (Turkington 1986) mentioned above: "Repeated use of designer drugs such as Ecstasy produces potentially irreversible brain damage." And an embarrassing elaboration of this misinformation was given in a newspaper interview, in which the following is a verbatim quotation from Dr. Charles Schuster (Associated Press 1986): "It can poison the nervous system probably irreversibly. It may very well be that a young, healthy adult who is exposed to these drugs is not going to show frank symptoms that are going to be picked up by a clinician. But what we don't know is whether 20 or 30 years from now, at the age of 45, they may begin to be showing central nervous system degenerative signs that ordinarily would not be seen until they get to be 70 or 80." It further quotes that this is the first demonstration of a neurotransmitter being modified to a neurotoxin. And from the NIDA bulletin: "MDMA — leads to psychotic episodes." All this is an equally inaccurate negative picture, without positives.