Abstract
Regiospecific alkoxylation of bromophenolic aldehydes has been carried out. Vanillin (I) is brominated (Br2/AcOH) to 5-bromovanillin (II) which is subsequently methylated to give 5-bromoveratraldehyde (III). The alkoxylation of II and III with freshly prepared sodium methoxide in the presence of anhydrous cupric chloride and dimethylformamide furnishes 4-hydroxy-3,5-dimethoxybenzaldehyde (IV) and 3,4,5-trimethoxybenzaldehyde (V) respectively. Methylation of IV with dimethyl sulphate and anhyd. potassium carbonate in dry acetone gives V.
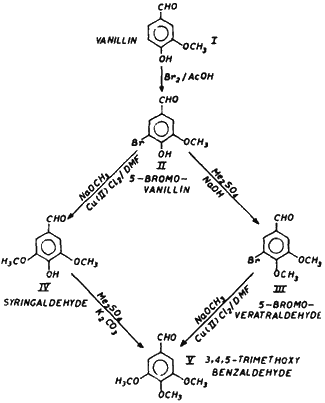
In our earlier work on lignins1-5 we have observed that the softwood lignin on oxidation gives vanillin (I) whereas hardwood lignin gives vanillin (I) and syringaldehyde (II). We have also reported3 a chemical method for the separation of I and II obtained as a mixture from hardwood oxidation reactions. Syringaldehyde was subsequently methylated to 3,4,5-trimethoxybenzaldehyde (V) which is an important drug intermediate used in pharmaceutical industry. It is commonly prepared by Rosenmund reduction6 of 3,4,5-trimethoxybenzoyl chloride. We now report a novel regiospecific alkoxylation procedure for the preparation of V from I which can be exploited on commercial scale.
Vanillin (I) was brominated in glacial acetic acid with bromine to give 5-bromovanillin (II). A portion of II was methylated to 4-hydroxy-3,5-dimethoxybenzaldehyde (III) with dimethyl sulphate and sodium hydroxide. The bromo compounds II and III were subjected to alkoxylation using methanolic sodium methoxide in the presence of cupric chloride and dimethylformamide to give 4-hydroxy-3,5-dimethoxybenzaldehyde (IV) and V, respectively. The reaction was conducted in a sealed glass ampule which was placed in an autoclave at 120°C. Compound IV on methylation with dimethyl sulphate and anhyd. K2CO3 gave V. It has been observed that alkoxylation of II was more facile than that of III. This may be attributed to the presence of a bulkier group at the ortho-position of III.
Experimental
Melting points reported are uncorrected. Rf values refer to TLC using C6H6-EtOAc (8:2) as eluent.
5-Bromovanillin (II)
Bromine (44.0 g) was added dropwise with stirring to a solution of vanillin (38 g) in glacial acetic acid (150 ml) for 2 hr the reaction mixture poured in ice-cold water (300 ml), and the precipitated solid filtered, washed with water and crystallised from 95% ethanol to give light yellow crystals of II, yield 48.5 g, mp 163-164°C (lit.7 mp 163-164°C), Rf 0.61.
5-Bromoveratraldehyde (III)
Dimethyl sulphate (80 ml) was added slowly to a stirred solution of II (21.0 g) in 2.5% sodium hydroxide solution (150ml) while keeping the temperature between 30-35°C. After the addition was over the solution was boiled for 2 hr, cooled in an ice-bath, and the precipitated product filtered and crystallised from 80% ethanol to get III as flocculant crystals, yield 1 g, mp 64-65°C (lit.8, mp 65-66°C), Rf 0.73.
4-Hydroxy-3,5-dimethoxybenzaldehyde (IV)
To a solution of II (23 g) in dimethylformamide (100 ml) and sodium methoxide (freshly prepared by dissolving 10g sodium in 200 ml super dry methanol) was added anhydrous copper(II) chloride (5.5g). The reaction mixture was sealed in a glass ampoule which was later put in an autoclave at 120°C for 1 hr. The reaction mixture was taken out and the excess of methanol distilled off. The residue was dissolved in water (200 ml), the aq. solution acidified with 50% HCl and extracted with chloroform. The organic layer after washing with water was dried (anhydrous sodium sulphate) and solvent removed in vacuo to give IV which crystallised from 95% ethanol, yield 18 g, mp 112-113°C (lit.10 mp 113°C), Rf 0.5.
3,4,5-Trimethoxybenzaldehyde (V)
- To a solution of IV (10 g) in dry acetone (50 ml) containing anhydrous K2CO3 was added dimethyl sulphate (9.2 g) and the mixture refluxed for 2 hr under anhydrous conditions. After the reaction was complete as monitored by TLC, the product was filtered and acetone distilled off. The solid residue was crystallised from 95% ethanol to give V as long needles (9 g), mp 73-74°C (lit.9, mp 74°C), Rf 0.52.
- The reaction of III was conducted under similar conditions as in the case of methoxylation of II. The residue after work-up and crystallization from 95% ethanol gave V, mp 72°C, yield 9g (76.5%) identical (mp, mmp, IR) with a sample of V obtained above.