Now I was wandering about the internet the other day and was thinking about something entirely different (Morphine synthesis from two adjacent 6-membered rings, ala "Pummerer's Ketone", strangely enough thinking about that is what led to the biomimetic synthesis of morphine) and for some strange reason had a look at limonene & THC...
That caused me to have a look at Cannibidiol (which apparently has the fortunate habit of cyclizing to THC in acid media), which essentially limonene attached via the ring to 3,5-Dihydroxy-1-pentylbenzene (at the p-position of the benzene ring).
Ok - so how to get to there? The wiki article on Terpineol may well provide the answer (provided the Pummerer-type route is open & I can't see why it shouldn't be).
The two oxygens - the one from the a-Terpineol and either of the phenolic hydroxyl's (look at the molecule - whichever one it binds to will give the same product) ala Pummerer ie. limonene + the 3,5-dihydroxy-1-pentylbenzene should react, in acid media, to give the ketone (given that the cannibidiol & limonene both give the wanted a-terpineol derivative in acid kinda supports that for mine). Once the -O- bond is formed then the rest should be relatively simple (if not exactly OTC or kitchen chemistry), as with morphine & Pummerer's ketone, once that -O- bridge is formed, oxidative cyclization (per Szantay, et al) or cyclization with strong acid (per Rice, et al) should give an effective (and hell shorter) route to THC. That would obviate the clunky as fuck technical synthesis that I've seen floating around online (erowid type).
Wouldn't that be interesting? Actual THC in a short-synthesis that is easier than that of the cannabinoid-receptor-ligands.
That caused me to have a look at Cannibidiol (which apparently has the fortunate habit of cyclizing to THC in acid media), which essentially limonene attached via the ring to 3,5-Dihydroxy-1-pentylbenzene (at the p-position of the benzene ring).
Ok - so how to get to there? The wiki article on Terpineol may well provide the answer (provided the Pummerer-type route is open & I can't see why it shouldn't be).
The two oxygens - the one from the a-Terpineol and either of the phenolic hydroxyl's (look at the molecule - whichever one it binds to will give the same product) ala Pummerer ie. limonene + the 3,5-dihydroxy-1-pentylbenzene should react, in acid media, to give the ketone (given that the cannibidiol & limonene both give the wanted a-terpineol derivative in acid kinda supports that for mine). Once the -O- bond is formed then the rest should be relatively simple (if not exactly OTC or kitchen chemistry), as with morphine & Pummerer's ketone, once that -O- bridge is formed, oxidative cyclization (per Szantay, et al) or cyclization with strong acid (per Rice, et al) should give an effective (and hell shorter) route to THC. That would obviate the clunky as fuck technical synthesis that I've seen floating around online (erowid type).
Wouldn't that be interesting? Actual THC in a short-synthesis that is easier than that of the cannabinoid-receptor-ligands.







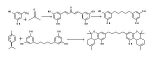
 Delta-9-THC via Pummerer's Ketone. Well... metabolites, close enough.
Delta-9-THC via Pummerer's Ketone. Well... metabolites, close enough. 
 I would post some too if I would be running something novel, which I'm not at the moment. Maybe later this summer
I would post some too if I would be running something novel, which I'm not at the moment. Maybe later this summer 