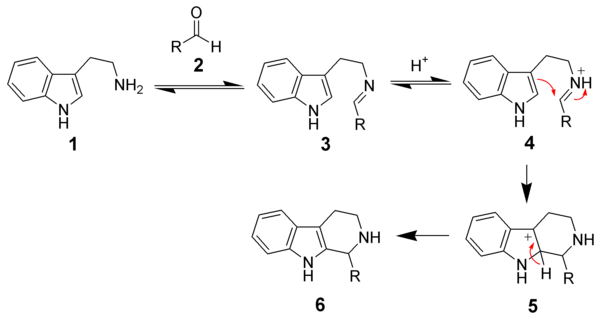
Being the idiot I am I just read someones experimental here about a test with beta-alanine.
The experimental details did not mention the use of a ketone catalyst, thus it was not employed.
A suitable catalyst is procured and the experiment rerun.
The experimental details did not mention the use of a ketone catalyst, thus it was not employed.
A suitable catalyst is procured and the experiment rerun.