Quote from the patent here!
http://www.google.com/patents/about?id=M3GIAAAAEBAJ&dq=tryptamine+formic+paraformaldehyde"The solvent is removed and the products are reacted with aqueous formaldehyde or a formaldehyde equivalent, such as paraformaldehyde, and a reducing agent, such as formic acid or sodium cyanoborohydride, to give N,N-dimethyltryptamine derivatives of formula I wherein X is hydrogen, R 1 N(CH 3 ) 2 and R 2 to R 5 are as set forth above."
A bee by the name of Rhodium wrote this regarding N,N-dimethylation of Tryptamine with formaldehyde and cyanoborohydride and why it is favored over borohydride;
"Note by Rhodium: The Pictet-Spengler side-reaction occurs whenever a reductive amination of Tryptamine is performed under acidic conditions (such as HCOOH/HCHO methylation), the reason sodium borohydride usually is disfavored is because it is powerful enough to reduce the formaldehyde to methanol before it has any chance to react with the tryptamine (unless suitable measures are taken to prevent this from happening)."
from here!!
http://www.erowid.org/archive/rhodium/chemistry/tryptamine2dmt.htmlSo the Pictet-Spengler is not some secret that no one knows about, and what I am proposing is not revolutionary at all! The above link is over 15 years old!!!! Formic acid is the only change and because it is also a reductant by virtue of the fact it is also hydride source just like cyanoborohydride or borohydride, not to as great an extent but still a hydride source never the less. If you still think it is revolutionary check the references on rhodiums page dated 1993. Also if 80%+ yields of N,N-dimethylated tryptamine are traces then wow!
Abstract from the paper on N-methylation with paraformaldehyde and oxalic acid;
"Primary and secondary amines are N-methylated by a mixture
of paraformaldehyde and oxalic acid dihydrate in good to
excellent yields. The reaction proceeds without involvement
of organic solvents and toxic formalin. Reaction temperatures
of 100*C are required for the decomposition of oxalic acid
into the intermediate formic acid which acts as the actual
reductant. The reaction conditions have been optimized, and
the mechanism has been elucidated by means of deuteration
experiments."
A SOLVENT-FREE AND
FORMALIN-FREE ESCHWEILER-CLARKE
METHYLATION FOR AMINEShttp://www.sciencemadness.org/talk/files.php?pid=86049&aid=2349Did that sound familiar?
"Reaction temperatures
of 100*C are required for the decomposition of oxalic acid
into the intermediate formic acid which acts as the actual
reductant."
Compared to the patent;
"products are reacted with aqueous formaldehyde or a formaldehyde equivalent, such as paraformaldehyde, and a reducing agent, such as formic acid or sodium cyanoborohydride, to give N,N-dimethyltryptamine derivatives"
Compared to Rhodiums page;
"Tryptamine (1.12g, 7 mmol), Sodium Cyanoborohydride (0.88g, 14 mmol) and Glacial Acetic Acid (2ml, 35 mmol) was dissolved in 110ml Methanol at 0°C, and a solution of 37% Formaldehyde (1.4 mL, 18.5 mmol) in 15ml Methanol was added dropwise over 20 min, and the resulting solution was allowed to stir for 20 min at 0°C and 2.5h at room temp. The methanol was evaporated under reduced pressure, and 80ml 25% aqueous potassium carbonate was added and the solution extracted with 2x125ml EtOAc, the extracts washed with 2x40ml brine, dried over MgSO4and the solvent evaporated under reduced pressure to give an amber oil, which was purified by flash chromatography on 30g silica gel (using a gradient of EtOAc:MeOH) to give an oil, which was crystallized from boiling hexane to give N,N-Dimethyltryptamine as colorless waxy crystals, weighing 0.9g (69%)."
that was taken from;
Jose L. Castro, et. al., J. Med. Chem. 37(19), 3023-3032 (1994)
ZZ comment;
"I mean they did not use formic acid at all, and it was not a methylation reaction. "
first sentence of the quote from the book;
"In the competition between Pictet-Spengler ring closure and Eschweiler methylation of tryptamine derivatives on treatment with formaldehyde in formic acid"
ZZ comment;
"Formiate anion is indeed the source of hydride, but i suppose sodium formiate can not be used as a reducing agent in E-C instead of formic acid. Because imine should also be protonated
http://en.wikipedia.org/wiki/Eschweiler-Clarke_reaction The only case when we can at the same time have formiate anion and protonated imine, is when the reagent is formic acid"
The iminium ion is not protonated! The first step of Eschweiler-Clarke methylation is a Mannich reaction and it is the aldehyde that picks up a proton and gains positive character which is why it is attracted to the electron rich amine! Once iminimium ion is formed hydride reduces it to an amine. Hydride sources are zinc hydride, sodium cyanoborohydride and formic acid. The mannich reaction can proceed in the solid state with the paraformaldehyde, oxalic acid and amine reaction because the oxalic acid dihydrate works as the acidic catalyst, it has to be the dihydrate because it acts as the hydrogen donor aswell. The yields are generally quantitative for this procedure.
note for ZZ;
I ask you, do you concede that tryptamines can undergo Escweiler methylation with sodium cyanoborhydride and formaldehyde?
As documented and referenced here;
http://www.erowid.org/archive/rhodium/chemistry/tryptamine2dmt.htmlbtw; The above rhodium link uses exactly the same mechanism as the reaction using formic acid but this time formic acid is the reductant (hydride source), not sodium cyanoborohydride.
Lilienthal's Pharmacological & Chemical References at;
http://westwood.fortunecity.com/storey/116/referenc.htmHas at least five examples like this;
5-subst. N,N-dimethyltryptamines from tryptamines with formaline, acetic acid, and NaCNBH4, yield 71% / 83%, p-CN-phenylhydrazine, Cl-butyraldehyde acetal reaction #152 J. Med. Chem.




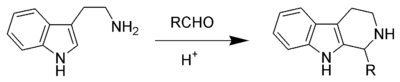



 Eshweiler methylation is a different reaction
Eshweiler methylation is a different reaction Although the mechanism is very close, Mannich base formation followed by reduction of the imine by hydride to an amine. Whereas the indole Pictet-Spengler, does the same acid catalysed Mannich base formation of the imine. But the imine is protonated to form an iminium ion which is prone to cyclization.
Although the mechanism is very close, Mannich base formation followed by reduction of the imine by hydride to an amine. Whereas the indole Pictet-Spengler, does the same acid catalysed Mannich base formation of the imine. But the imine is protonated to form an iminium ion which is prone to cyclization.