I have been studying these lately, there is an alkene double bond just like isosafrole, there are several fairly simple routes for the epoxidation of either of these. I will post pictures below.
I searched this whole forum, this is the only cinnamic thread so im not covering old ground
This is in cinnamon 80%.. it is very common stuff
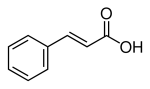
If Cinnamic acid was epoxidised, then reformed to a ketone structure with sulfuric (the same as in the peracid route to mdp2p), then if this worked up as though it was p2p, so a reductive amination via Al Hg amalgum, i want to know where the NH2 would land? i believe carbox acids are 'protected' i am thinking the amination will take place in the same way, then from the aminated product the key groups are fairly resistant to many elimination reactions like clemmensen or wolf kishner..
There are several methods of eliminating an aldehyde function, 1 method is to brominate, which will replace the aldehyde function and then react that with NaOH which will steal the bromine away leaving a carbon. there are simpler elimination methods i am sure you guys would know them
The key would be to be able to eliminate the aldehyde function with out destroying that double bond between the second and third carbon.. Most elimination reactions ive seen on cinnamic leave Phenyl Ethanol, so the double bonded carbon get sacrificed..
Anyone have ideas here?
the reagents used below arnt very OTC but its an example of the structure change





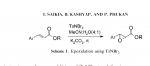
 Cinnamaldehyde can be reduced by aluminum isopropoxide using the Meerwin-Ponndorf reduction as in Ann 444 221 (1925) to cinnamyl alcohol
Cinnamaldehyde can be reduced by aluminum isopropoxide using the Meerwin-Ponndorf reduction as in Ann 444 221 (1925) to cinnamyl alcohol  As far as cinnamyl alcohol:
As far as cinnamyl alcohol:
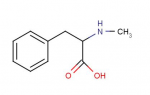


 there seems to be some 'conflicting info here'!
there seems to be some 'conflicting info here'! 