Abstract
The two-carbon homologs of two potent psychotomimetic agents are described. Unlike the parent isopropylamine compounds (4-methyl-2,5-dimethoxyamphetamine, DOM, STP; and 4-bromo-2,5-dimethoxyamphetamine, PBR, 4-BR) these phenethylamines lead to an intoxication state which is, normal subjects, of short duration and of greatly increased sensory enhancement, but which does not superimpose hallucinogenesis. These two phenethylamines, 4-methyl-2,5-dimethoxyphenethylamine (II) and 4-bromo- 2,5-dimethoxyphenethylamine (III), are active in man at oral levels of 0.1 to 0.2 mg/Kg, approximately one-tenth the potency of their three-carbon counterparts.
Introduction
Although mescaline (I) has served as a model for a number of studies inquiring into the structure-activity relationships of psychotomimetic drugs1, most of the compounds studied have contained the three-carbon chain characteristic of the stimulant amphetamine. In those instances where a direct comparison has been made between identically substituted 2- and 3-carbon chain. derivatives, the 3-carbon homologs are quantitatively more potent and are qualitatively of a more severe and commanding nature.
These characteristics are reminiscent of LSD, but are distinct from the euphoric and benign nature usually associated with mescaline1,2,3. Exceptions to this generality are to be found in the two methylenedioxy-bases MDA4 and MMDA5 in which the induced intoxication state is not of the "psychotomimetic" character usually associated with the 3-carbon chain but rather more similar to the mescaline model. The 2-carbon homologs of these latter two drugs are known but have not been explored pharmacologically.
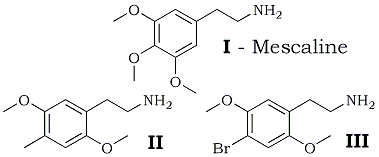
The continuing studies of ring-substitution patterns and the evaluation of the resulting compounds in human volunteers have revealed two highly potent drugs, 4-methyl- 2,5-dimethoxyamphetamine (DOM, STP)6 and 4-bromo-2,5-dimethoxyamphetamine (PBR, 4-BR)7. The present report describes the synthesis and pharmacology of the 2-carbon counterparts of these two materials; viz., 4-methyl-2,5-dimethoxyphenethylamine (II) and 4-bromo-2,5-dimethoxyphenethylamine (III).
Materials and Methods
4-Methyl-2,5-dimethoxyphenethylamine (II) was prepared by the chemical interaction of 2,5-dimethoxy-4-methylbenzaldehyde and nitromethane, followed by the reduction of the resulting nitrostyrene by LiAlH4. It is isolated as the hydrochloride salt, a white solid with melting point 211-212°C after recrystallization from ethanol (lit.8 mp 212-213°C). 4-Bromo-2,5-dimethoxyphenethylamine (III) was obtained from 2,5-dimethoxyphenethylamine by elemental bromination in acetic acid as has been described for the corresponding phenylisopropylamine7. This product was also obtained as the hydrochloride salt with a residual pale pink color which persisted despite recrystallization from ethanol. This can be removed by heating the free base in 25% NaOH. The melting point of the hydrochloride salt is 237-239°C, with decomposition.
Human potencies and qualitative descriptions of activity were determined by the "double-conscious" technique1,9, With each drug (II and III) we used the same ten subjects, all between 23 and 35 years of age. Six of the subjects had had some experience with hallucinogens, but we accepted no one who had used any hallucinogen more than twice in the preceding year. The setting was non-therapeutic, comfortable, and with music available if wanted. All subjects were tested with two observers present. Threshold dose was determined by starting at 0.5 mg, and increasing in increments of 0.2 mg. At least five days elapsed between the same subject receiving subsequent doses. Five of the subjects were used for a single experiment each above 10 mg for III and 12 mg for II.
Results and Discussion
Threshold effects are noted with 4-methyl-2,5-dimethoxyphenethylamine (II) at approximately 6 mg (total dose, oral) and a fully intense intoxication follows the administration of 10 to 15 mg. Onset of subjective changes are noted in 20 to 30 minutes at these latter doses, peak effects are achieved at between 1.5 and 2 hours and are clearly subsiding after another hour. Mydriasis is noted only following doses in the range of 15 to 25 mg and although the intensity of the subject effects appears to be increased the chronology of intoxication remains unchanged. 4-Bromo-2,5-dimethoxyphenethylamine (III) displays approximately the same threshold level (4 mg, oral) and is slightly more potent than II with an effective dose range of 8 to 10 mg. The duration of action (6 to 8 hours) at this latter level is somewhat longer than that observed (5 to 6 hours) for II.
Preliminary observations at fully effective doses indicate the following subjective results. Both of these bases lead to a similar state of intoxication, one quite separate and distinct from that usually associated with the "psychotomimetic" or "hallucinogenic" drug. Rather than showing signs of stimulation, the subject becomes passive and relaxed and is aware of an integration of sensory perception with emotional state (although not experiencing the lassitude characteristic of psilocybin). Except for the consistent perceptual enhancement noted, there are no effects that can be called hallucinogenic. At these dose levels there is a considerable euphoria with an increased body awareness and increased receptiveness of visual, auditory, olfactory, and tactile sensation. The integration of sensory and emotional states induces in most subjects a feeling of security and an ability to cope with incidents and experiences that might have led, with drugs such as LSD, to a state of anxiety and possible panic. Our observations also suggest that at equivalent doses based on threshold doses the effects of II are more intense in most respects than those of III. At the end of the experience (6 hours for II, 8 hours for III) the subject appears to be alert, relaxed, and content.
There is a recognized need for psychotropic agents which can be employed in psychotherapy and psychological research, to free a patient from a reluctance to respond to the omnipresent stimuli about him but not at the cost of superimposing upon him a clinically unacceptable state of hallucination or inability to communicate. We believe that these two chemicals may serve as possible experimental models in this area of research and therapy.