Abstract
A variety of aromatic compounds substituted with an electron donating group such as methoxy, hydroxy, or amino group, were regioselectively iodinated with iodine in the presence of tetrabutylammonium peroxydisulfate under mild conditions in excellent yields.
Iodoaromatic compounds have long been employed in organic synthesis,1a because they can be readily functionalized through carbon-carbon bond formation of diarenes, ethylenic or acetylenic condensations using transition metals or carbon-heteroatom bonds.1b The iodination of aromatic compounds has been the subject of numerous studies due to a potential ability as intermediates in organic syntheses and also as bacterial and fungicidal agents.2 The direct introduction of iodine into aromatic molecules under mild conditions needs some additive to activate the low reactivity of iodine. In recent years, the direct iodination methods have been intensively developed by using iodonium donating systems, such as iodine-nitrogen dioxide,3 iodine-F-TEDA (1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)),4 NIS (N-iodosuccinimide),5 iodine-diiodine pentoxide,6 iodine-mercury(II) oxide,7 iodine monochloride,8 bis(pyridine)iodonium(I) tetrafluoroborate-CF3SO3H,9 NIS-CF3SO3H,10 iodine-silver sulfate,11 iodine-mercury salts,12 NaOCl-NaI,13 iodine-Na2S2O8,14 and iodine-(NH4)2S2O8-CuCl2-Ag2SO4.15 However, most of these methods require hazardous or toxic reagents, or high reaction temperature for a long reaction time.
Scheme 1
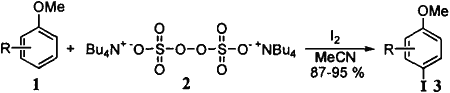
We have found a practical and regioselective aromatic iodination: a combination of tetrabutylammonium peroxydisulfate ((TBA)2S2O8) and iodine has been found to be an excellent reagent for the efficient iodination of aromatic compounds such as methoxybenzene, phenol, and aniline in acetonitrile under mild conditions (Scheme 1). This reaction has an advantage to be carried out at 20°C under the neutral condition in acetonitrile.
Tetrabutylammonium peroxydisulfate was successfully prepared16c and turned out to be a useful source of tetrabutylammonium sulfate radical, which can be readily converted to sulfate anion by one electron transfer from a substrate.16
Run | Substrate (1) |
Time | Temp | Product (3) |
Yielda |
1 |
0.7 h | 20°C | 95% | ||
2b |
1 h | 60°C | 85% | ||
3 |
3 h | 20°C | 92% | ||
4 |
 | 6 h | 50°C |
 |
92% |
5 |
 | 7 h | 50°C |
 |
93% |
6 |
 | 7 h | 50°C |
 |
87% |
7 |
 | 2 h | 20°C |
 |
91% |
8 |
 | 20 h | 20°C |
 |
87% |
9 |
 | 24 h | 20°C |
 |
88% |
10 |
 | 30 h | 20°C |
 |
92%c |
11 |
 | 20 h | 20°C |
 |
93% |
- lsolated yield.
- ln run #2, 0.5 eq. of each (TBA)2S2O8 and I2 was used.
- NMR yield.
In order to generalize the new iodination reaction, a variety of methoxy arenes, phenol or aniline derivatives were submitted to the reaction with 12 in the presence of (TBA)2S2O8 at 20°C in acetonitrile.17 The products obtained in all cases resulted in the regioselective p-iodination toward -OMe, -OH, and -NMe2 of the aromatic ring. The results obtained are summarized in Table 1. The regioselectivity may be deduced from the result that iodination occurs at a more electron-rich and less sterically hindered position. Thus, p-positions of methoxybenzenes (runs 1, 4-7, and 11 in Table 1)) and phenol (run 10 in Table 1) were exclusively iodinated (runs 1, 4, 5, 6, 7, 9, 10, and 11 in Table 1), whereas o-iodination only occurred when the p-position was blocked with a substituent (runs 3 and 8 in Table 1). The reactivity of the substrates seems to be associated with the electron density of the aromatic rings. Iodination of dimethoxybenzenes took place at room temperature in a short time (1-7 h), whereas the reactions of monomethoxybenzenes required 6-20 h. In the case of m-methoxyanisole (run 1 in Table 1), iodination occurs more rapidly than p-dimethoxybenzenes (run 3 in Table 1). This result indicates that p-orientation is preferred over o-orientation. Although the iodination of anilines and phenols is slow compared to that of methoxybenzenes, anilines and phenols were well iodinated in good yields under the same reaction conditions. The iodination of acetophenone or nitrobenzene is too slow under the same reaction conditions: Starting material was recovered quantitatively.
Scheme 2
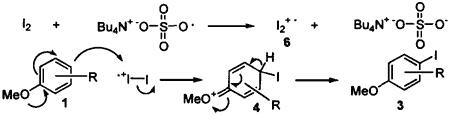
Possible mechanism for aromatic iodination
Scheme 3
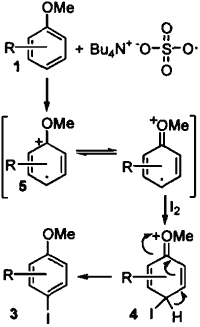
The reaction mechanism is not clear, but it may be explained by one of the two possible pathways. The sulfate free radical produced by homolytic cleavage of (TBA)2S2O8 may oxidize iodine via a one electron transfer to produce the iodonium cation radical 6. The electrophilic attack of the iodonium cation radical at the p-position of methoxybenzene produces the intermediate 4, which is rapidly oxidized to the iodinated methoxybenzene 3 (Scheme 2). Another alternative pathway is that the sulfate free radical oxidizes methoxybenzene by one electron transfer from it to produce the phenyl cation radical 5 that reacts with iodine to form the adduct intermediate 4, followed by oxidation to 3 (Scheme 3). The scope and reaction mechanism is under investigation.