 |
|
|
||||||||||||||||||||||||||
|
Recent advances in pethidine-type analgesicsSectionsReplacement of the N-methyl Group of Pethidine and Related Compounds by other Groups DetailsAuthor: A. H. Beckett , A. F. Casy Recent advances in pethidine-type analgesicsA. H. Beckett School of Pharmacy, Chelsea College of Science and Technology, London, S.W.3A. F. Casy School of Pharmacy, Chelsea College of Science and Technology, London, S.W.3 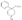 Synthetic analgesics including substances related to pethi-dine[1] have recently been comprehensively reviewed by Braenden, Eddy & Halbach (1). It is the purpose of this paper to describe important pethidine-type compounds that have been reported since this review and to present detailed information concerning 1: 3-dialkyl-4-aryl-4-acyloxypiperidines and 7-membered ring analogues of pethidine. A study of structure-activity relationships in this field will also be made. Replacement of the N-methyl Group of Pethidine and Related Compounds by other GroupsUntil recently it was thought that, for optimum activity, the basic group of an analgesic must bear a methyl group and that substitution of this group by higher alkyl, or other groups, must result in a fall in activity ([1] ). That this is not the case has now been shown by the synthesis of several analgesics substituted by the N-2-phenylethyl group (and other groups) that have greater activities than the corresponding N-methyl compounds. Thus N-2-phenylethyl- normorphine (I) and nordromoran (II) are more potent analgesics than their respective parent compounds ([2] , [3] ). Perrine & Eddy ([4] ) report that N-2-phenylethyl and N-2-hydroxy-2-phenylethyl norpethidine (III and IV respectively, see flow sheet 2), are both at least twice as active as pethidine. Acetylation of the hydroxy compound giving (V) results in an almost complete loss of activity. Weijlard et al. ([5] ) have synthesized N-2- p-aminophe-nyl norpethidine (anileridine, VI) and state that it has several times the activity of pethidine. In a more detailed study, Orahovats et al. ([6] ) report that anileridine is ten to twelve times more potent than pethidine in animals, with high oral activity and relatively mild side reactions. May ([7] ), in contrast to the above results, has found that replacement of N-methyl by N-2-phenylethyl in a number of morphine-fragment type compounds (VII and VIII) results in a fall in analgesic potency. Anderson, Frearson & Stem ([8] ), have prepared a series of pethidine analogues of type (IX) in which Y represents an alkyl group carrying a heterocyclic residue X. Several of these compounds show notable activity, and one, morpholinoethyl norpeth  times more potent than pethidine itself ([9] , [10] ). When oxygen is replaced by sulphur in the heterocyclic residue  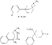
Table 1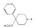 pethidine whereas compounds lacking oxygen or sulphur in  inactive. Lengthening or shortening of the carbon chain linking the two nitrogen atoms of the two rings results in a considerable reduction of activity; branching also reduces activity, though not sharply. Elpern et al. ([66] ) have recently reported an extensive series of 1-aralkyl-4-carbethoxy-4-phenylpiperidines and obtained the following results:
SynthesisMost of the compounds described in the previous section were prepared by direct N-substitution of norpethidine (X) --an intermediate in one of the commercial methods for the manufacture of pethidine. Flow sheet 1 indicates the synthesis of this intermediate[2] and flow sheet 2 summarizes the synthesis of some of the compounds listed in table 1. Reaction of the secondary base (X) with phenylethyleneoxide gives 4-carbethoxy 1-(2-hydroxy-2-phenylethyl)-4-phenylpiperidine (IV) which on hydrogenation gives N-2-phenylethyl norpethidine (III) and on acetylation, the ester (V). Weijland et al. ([5] ) obtained the 2- p-aminophenylethyl analogue (VI) by alkylation of norpethidine with p-aminophe-nylethylchloride hydrochloride in the presence of alkali. The compounds of Anderson et al. ([8] ) were obtained by treating norpethidine with the appropriate substituted alkyl halide-e.g., 2-morpholinoethylchloride to give (XI). Where this method proved difficult (compounds 10, 13 and 14 in table 1) the secondary base was first alkylated with ethylene chlor-hydrin and the resultant 2-hydroxyethyl compound (XII) converted into the 2-chloroethyl compound (XIII) which reacted readily with heterocyclic bases - e.g., piperazine to give (XIV). The majority of the compounds of Elpern et al. ([66] ) were prepared by alkylation of norpethidine with the appropriate alkyl halide; the pyridylethyl compounds were obtained by condensing norpethidine with 2- and 4-vinyl-pyridine respectively, and N-(3-phenyl-2-propargyl) ethyl- norpethidine by treatment of the secondary base with formaldehyde and phenylacetylene. Flow sheet 1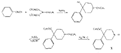
Flow sheet 2 (Synthesis of certain of the compounds listed in table 1)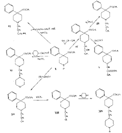
Reversed Esters of PethidineIn 1943, Jensen et al. ([11] ) found that replacement of the carbethoxy group of pethidine by the propionoxy group (-OCOC 2H 5) resulted in a fivefold increase in activity over that of the parent compound. Independently, workers in the Roche Laboratories ([12] , [13] ) prepared many compounds of this type (the so-called "reversed esters of pethidine ") and confirmed that the propionoxy compound (XV, R = C 2H 5) gave the most active member of the series. 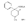 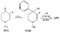 The Roche workers found that the substitution of a methyl group into the 3-position of the piperidine ring resulted in a further increase in potency ([14] ). The compound (XVI, R = CH 3) was obtained in two geometrically isomeric forms, designated alpha-and betaprodine respectively, the latter isomer being resolved into its optical enantiomorphs ([29] ) (for configuration of these isomers, see p. 44). Randall & Lehmann ([14] ) obtained the following pharmacological results in rats, morphine being taken as 100, &alpha-form racemate 97, &beta-form racemate 550, (+)-&beta-form 350, |( - )-&beta-form 790. In man, the difference in action between the &alpha-and &beta-racemates is not so pronounced ([16] ). Gross et al. ([17] ) investigated alpha-and betaprodine in man, and Houde et al. ([18] ) reported that alphaprodine had a weaker analgesic action than morphine and showed side effects in 10% of the patients. The use of alphaprodine as a postoperative analgesic has been investigated by Bachrach et al. ([15] ). Evidence that these substances show addiction properties has been obtained by Isbell ([19] ). Janssen ([20] ) has recently determined the analgesic activity of prodine isomers prepared in our own laboratories and obtained the following results, morphine hydrochloride being taken as 100; in mice, &alpha-form racemate 200, &beta-form racemate 835, in rats, &alpha-form racemate 190, &beta-form racemate 3000. Randall & Lehmann ([14] ) reported that the activity of 3-ethyl-1 -methyl-4-phenyl-4-propionoxypiperidine (XVI, R = C 2H 5) (no information concerning isomers is given) was 6.4 times that of morphine while the corresponding acetoxy ester possessed only half the latter's activity. Thus in analgesics of formula (XVI), activity increases in the order (XVI, R = H), (XVI, R = CH 3) and (XVI, R = C 2H 5) - i.e., with increasing size of the 3-substituent. McElvain ([21] ) has synthesized and tested the next higher member (XVI,R = n-C 3H 7) which is stated to have profound activity in rats at doses of 8 mg/kg, but no direct comparison has been made with the lower homologues and it is therefore not possible to draw any conclusions with regard to the optimal size of the 3-substituent from this evidence. Large groups in the 3-position such as benzyl completely abolish activity ([21] ) (see compounds 8-10, 14 and 15, table 2). The acetoxy and butyroxy esters of 1-methyl-3- n-propyl-4-phenylpiperidin- 1-ol are less active than the propionoxy ester, the latter again representing the most active member of a series. Inclusion of a double bond into the 3-substituent of XVI results in a highly active compound. This has been shown by the recent report of the analgesic activity of the 3-allyl analogue of prodine - i.e., 3-allyl-l-methyl-4-phenyl-4-propionoxypiperidine (XVI, R = CH 2CH = CH 2). Benson et al. ([69] ) have found that this compound is approximately ten times as active as alphaprodine with no corresponding increase in its toxicity [details of synthesis have become available subsequent to the submission of this paper for publication ([73)] ]. May ([70] ) has reported structural isomers of alpha- and betaprodine in which the 3-methyl and 4-propionoxy substituents are interchanged. These analogues possess very little analgesic activity. From information so far available, it appears that the 4-aryl substituent of prodine-type compounds must be a phenyl group if optimum activity is to be attained. In the case of alphaprodine, Randall and Lehman ([14] ) have reported substitution of 4-phenyl by 4-cyclohexyl to result in a considerable fall in activity (see compound 5, table 2), while work in our own laboratories ([30] ) has shown that replacement by p-tolyl, o-tolyl and m-tolyl gives progressively less active substances (see compounds 25, 18 and 22, table 2). Synthesis The key intermediates in the synthesis of compounds of type (XVI) are 3-substituted 4-piperidones (XVII); reaction of the ketone (XVII)with an aryl lithium and subsequent acylation of the resultant tertiary alcohol (XVIII) gives the "reversed ester" (XVI). The 3-methyl substituted compounds listed in table 2 were prepared from 1: 3-dimethyl-4-piperidone (XVII, R = CH 3); Howton's preparation of this ketone, a modification of the usual 4-piperidone synthesis ([22] ), is outlined in flow sheet 3. Table 2
Flow Sheet 3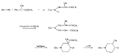 This method cannot serve as a general procedure for the preparation of higher 3-alkylhomologues as the necessary substituted acrylic esters are not readily available. Treatment of 3-carbethoxy-l-methyl-4-piperidone (XIX) with an alkyl halide leads, as shown by McElvain ([23] ) and confirmed in or own laboratories, to an N- rather than a C-alkylated product. C-alkylation is possible once the basic nature of the nitrogen atom of the 4-piperidone is masked; thus McElvain ([23] ) has C-alkylated 1-benzoyl-3-carbethoxy-4-piperidone. The ketone (XIX) has been successfully C-allylated and benzylated by treatment with allyl and benzyldimethylanilinium bromide respectively ([21] ). Decarboxylation (prior reduction of allyl to propyl is necessary) of the products leads to the 3- n-propyl and 3-benzyl ketones (XVII, R = n-C 3H 7 and -CH 2C 6H 5 respectively), from which compounds 8-13 in table 2 were prepared. 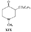 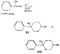 Recently, a new one-stage synthesis of the parent alcohol of the reversed ester of pethidine has been reported ([25] ). The method involves the amino-methylation of &alpha-methylstyrene by means of a methylamine hydrochloride-formalin mixture and gives the 4-piperidinol (XX) in 30% yield together with a substituted oxazine (XXI) as the major product. A modification of the method, employing N, N', N" -trimethyltrimethylenetriamine gives the reversed ester itself in 30% yield ([26] ); use of higher &alpha-substituted styrenes leads to prodine and its analogues ([67] ). Stereochemistry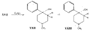 Treatment of the 4-piperidone (XVII) with phenyl lithium may give rise to two isomeric tertiary alcohols in which the C 6H 5 and R groups are respectively cis (XXII) and trans (XXIII). In the case of 1: 3-dimethyl-4-piperidone (XVII, R=CH 3), Ziering & Lee ([29] ) isolated two isomers by fractional crystallization of the propionic ester hydrochlorides. They named the two isomers alpha- and betaprodine respectively and assigned the cis (CH 3/C 6H 5) configuration to the alpha- and the trans(CH 3/C 6H 5) to the beta-compound. These assignments were not rigidly established and were stated to be based upon the easier break-down of the alpha-isomer under hydrolytic conditions and upon the pharmacological results, presuming the more active beta-isomer to be more closely related to the potent analgesic-dihydrodeoxy-morphine -D than the alpha-isomer as shown on the left ([14] ). 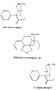 Conventional line representations, as above, may give a false indication of actual molecular shape; this can be derived with greater reliability by the aid of molecular models. To construct these, it is necessary to consider the possible conformations of the piperidine ring substituents. Application of the principles of conformational analysis leads to (XXIV) and (XXV) as the most probable conformations of the cis and trans isomers respectively (see Fig. 1). Comparison of these models with that of morphine (XXVI) (see Fig. 1) and a consideration of" fit" at a proposed receptor surface first led the present authors to question Ziering & Lee's configurational assignments ([27] ). Of the two isomers, the three-dimensional arrangement (XXIV, cis CH 3/C 6H 5) bears the greater resemblance to morphine and would therefore be expected to represent the more analgesically active isomer (betaprodine), while the less active isomer (alphaprodine) is represented by (XXV, trans CH 3/C 6H 5). Support for the reversal of the original configurational assignments is provided by hydrolysis studies on alpha- and betaprodine. The rate of hydrolysis of betaprodine has been shown to be greater than that of the alpha-compound ([28] ); the former must therefore have an equatorial (as in XXIV) and the latter compound an axial propionoxy group (as in XXV). Consideration of the isomer ratios obtained experimentally leads to the same conclusion - the original workers ([29] ) obtained an alpha : betaprodine ratio of 1.4:1, and Beckett et al. ([30] ) obtained 3: 1. The stereochemistry of addition of lithium aryls to the piperidone (XXVII) favours approach of the aryl group from the least hindered side of the molecule - i.e., attack from side ( b) wilt be preferred to attack from side ( a) (see XXVII), and in the product, the predominating isomer will have an equatorial aryl group. If these arguments are correct, increase in size of the aryl addendum should favour attack from side ( b) to an even greater degree. Our own results bear out this contention; treatment of the ketone (XXVII) with m- or p-tolyl lithium gives isomeric pairs of compounds in which one isomer predominates, whereas addition of the highly hindered o-tolyl or o-methoxyphenyl lithium results in the formation of one isomer exclusively. Evidence for the configurational identity of the isomers formed in major amount in the addition of phenyl lithium and m- and p-tolyl lithium to the piperidone (XXVII) and the single isomers formed upon addition of o-tolyl and o-methoxyphenyl lithium to this ketone, is provided by infra-red absorption measurements. The latter reveal a consistency of pattern for these isomers in the regions 990 to 1,020 cm-1, 1,350 to 1,385 cm-1 and 2,670 to 2,780 cm-1, which is completely different from that shown by isomers formed in minor amount upon addition of phenyl lithium and m- and p-tolyl lithium to the piperidone (XXVII). This evidence is presented in greater detail in a recent paper ([30] ). FIGURE 1 - Diagrammatical representation of the three-dimensional arrangement of analgesics and the "analgesic receptor surface".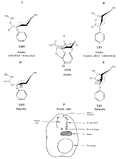 The diagrams represent the lower surfaces of the drug and the upper surface of the receptor- i.e., complementary surfaces. In front of, behind, and in the plane of the paper are represented by 0,........, and _______ respectively. 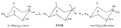
Azacycloheptanes related to Pethidine Glassman & Seifter ([31] ) have recently described the analgesic activity of a series of azacycloheptanes (hexamethyleneimines) related to pethidine. They found, in general, that replacement of the six-membered ring of pethidine and related compounds by a seven-membered one results in at least a 50% loss in analgesic potency. The straight pethidine analogue (XXVIII, compound I, table 3) is about one-third as active as pethidine; various modifications of the 4-phenylaza-cycloheptane molecule gave a number of compounds with analgesic potency equivalent to or better than that of pethidine. Changes in potency as a result of such modifications appear to parallel similar changes in the pethidine molecule itself. The most active compounds in this series were obtained by substitution of a methyl group in the 2 or 3 position of the ring (e.g., compounds 2, 3 and 7, table 3); substitution in the 5, 6 or 7 positions gave compounds with little or no activity (e.g., compounds 4, 5 and 6, table 3). Reversal of the carbethoxy group coupled with 3-methyl substitution gave the prodine analogue (compound 18, table 3). The latter was the most active member of the series being approximately ten times as active as pethidine; its configuration is provisionally reported as cis(CH 3/C 6H 5) ([32] ). Ketobemidone (1 - methyl - 4 - m - hydroxyphenyl - 4 - propionylpiperidine) is stated to be approximately thirty times as active as pethidine; the analogue in this series (compound 22, table 3) shows only a twofold increase over the pethidine analogue (compound 1, table 3). Although the latter is less active an analgesic than some of the other members of this series, its clinical use has been studied in detail as it appears to be without certain of the undesirable side-effects of morphine and pethidine ([33] , [33] and [35] ). Fraser ([71] ) reports that the addictive potentialities of the pethidine analogue and certain 1: 2 - and 1: 3-dimethyl derivatives (compounds 1, 3 and 7, table 3) are either low or non-existent, while the alphaprodine analogue (compound 18, table 3) has addiction liability equal to that of pethidine and approaching that of morphine. SynthesisSynthesis of 4-cyano-l-methyl-4-phenylazacycloheptane (XXIX) by condensation of the amine (XXX) with phenyl-acetonitrile - i.e., by a method analogous to the synthesis of pethidine cyanide, gave only a small yield of the desired product. Flow sheet 4 outlines the reactions finally employed by Diamond ([32] , [68] ) for the preparation of the cyanide (XXIX) and its conversion into the pethidine analogue (XXVIII) and the ketone (XXXI). A similar synthesis has been reported by Blicke & Tsao ([36] ). Flow sheet 4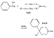 >The pethidine analogue (XXVIII) has been resolved via the menthyl ester diastereoisomers and also by crystallization of the (+)-acid tartrates. Diamond ([32] ) states that preliminary reports indicate that most of the analgesic activity of the racemic mixture may reside in one of the isomers. Modifications of the above synthesis employing m-methoxyphenylace-tonitrile and 2'-thienylacetonitrile gave the ketobemidone analogue (XXXII, compound 22, table 3) and 4-(2'-thienyl)-4 -carbethoxy- 1 - methylazacycloheptane (XXXIII, compound 23, table 3) respectively. 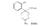 Condensation of 1 - dimethylamino- 2- chloropropane (XXXIV) with phenylacetonitrile yields a mixture of two isomeric cyanides (XXXV and XXXVI) probably owing to the intermediate formation of an ethyleneiminium salt (XXXVII) [c.f. the synthesis of methadone and isomethadone cyanides ([37] )]. 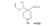 Application of the general synthesis (see flow sheet 4) to these two cyanides leads to the 1-2 dimethyl compounds (XXXVIII, XXXIX and XL, compounds 2, 7 and 13, table 3) and to the 1-3 dimethyl compound (XLI, compound 3, table 3); complications in the synthesis arise on account of the existence of diastereoisomeric forms. The 1-5 and 1-7 dimethyl compounds (XLII and XLIII, compounds 4 and 6, table 3) were obtained by condensation of the cyanide (XLIV) with crotonaldehyde and methyl vinyl ketone respectively. The products, after reduction to the alcohols, were treated with thionyl chloride to give the chlorocompounds (XLV and XLVI); the latter were used in the general synthesis as before. 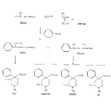 
TABLE 3Analgesic activities of Azacycloheptanes
Measured in rats by intraperitoneal injection. Flow sheet 5 outlines the reactions employed by Diamond ([32] ) for the synthesis of analogues of the reversed esters of pethidine. One method involves cyclization of the dicyanide (XLVII) by means of lithium ethylanilide (Ziegler's method) and the other two, replacement of the cyanide group of the precursor (XXIX) by the hydroxy or carbomethoxy group. These transformations were carried out in two ways: Hofmann degradation of the derived amide (XLVIII) to the amine (XLIX) followed by treatment with nitrous acid gave the tertiary alcohol (L); cleavage of the cyanide group with sodamide gave the hydrocarbon (LI), which on oxidation with lead tetraacetate gave the 4-carbomethoxy compound (LII). Application of the latter method to the cyanides (XXXV and XXXVI) gave compounds 15 and 16 in table 3. The cyanoester (LIII), obtained by condensation of the secondary amine (LIV) with &gamma-chloro- n-propylcyanide, underwent a Dieckmann condensation on treatment with sodium methoxide; the resultant mixture (LV and LVI), on heating with hydrochloric acid, gave 1:3-dimethyl-4-azacycloheptanone (LVII), from which was derived the prodine analogue (LVIII, compound 18, table 3; see flow sheet 6). The latter compound is believed to be one pure diastereoisomeric form (the other isomer has not been isolated); its configuration is pro visionally reported as cis (CH 3/C 6H 5) on the basis of pyrolysis studies.[3] Flow sheet 5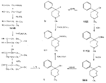
Flow sheet 6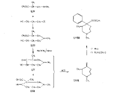
Note on PromedolNazarov has reported the synthesis of a series of 4-piperidinol esters derived from 1-alkyl-2 : 5-dimethyl-4-piperidone (LIX). Ketones of this type are obtained from technically available dimethylvinylethinylcarbinol (LX) as indicated in flow sheet 7 ([38] ). Flow sheet 7 The ketone (LIX) may exist in cis and trans forms, but attempts at isomer separation in the case of (LIX, R = H) have yielded only one form (probably the trans isomer) (39). Promedol, 1 : 2: 5-trimethyl-4-phenyl-4-propionoxypiperi-dine (LXI, R = CH 3, R' = C 2H 5) is one of the isomers obtained by treatment of the ketone (LIX, R = CH3) with phenyl lithium and acylation of the resultant tertiary alcohols ([40] ). The analgesic activity of promedol is reported to be several times greater than that of pethidine ([41] , [42] ). Further details of the stereochemistry of esters of 1: 2: 5-tri-methyl-4-phenylpiperidinol have recently been published ([43] ); a stereoisomer, isopromedol, is reported to be 2 to 3 times more active than promedol and has been approved for clinical use in the USSR ([44] ). Further details concerning promedol have been given in a recent paper in this bulletin ([72] ). The Relationship of Structure to Analgesic ActivityIt has been suggested that the activity of pethidine-type analgesics lies in the fact that the 4-phenylpiperidine ring system represents an essential fragment of the morphine nucleus and that the three-dimensional structure of the latter is simulated more or less closely by the various active compounds ([45] ). The azacycloheptanes of Diamond ([32] ) may similarly be considered as morphine fragments (see formulae LXII and LXIII). Recent workers have placed less emphasis upon the need for the piperidine ring as such and have stressed the importance of over-all spatial configuration ([46] , [47] ). 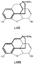 The decisive role of stereochemical factors in analgesic action is demonstrated by ([1] ) the many examples of enantiomorphic pairs in which most of the activity resides in one of the isomers; ([2] ) the considerable differences in activity amongst the prodine isomers; and ([3] ) the relationships between analgesics and their antagonists- thus morphine and nalor- phine [N-allyl normorphine] have identical configurations; levorphan, a potent analgesic, is antagonized by (-)-3-hydroxy -N-allylmorphinan of identical configuration, but not by the (+)-allyl, compound possessing the same configuration as the almost inactive dextrorphan ([27] ). The present authors have obtained further evidence for the configurational requirements of analgesics by relating the configuration of a number of the more analgesically active isomers of enantio-morphic pairs to D-(-)-alanine ([48] , [49] and [50] ). From this evidence they postulated that, for an organic compound to exhibit high analgesic activity, the following essential features are necessary:
Active analgesics were shown to have structures which enable them to present similar surfaces to allow of their association with a proposed "analgesic receptor surface" (see Fig. 1). Both pethidine (pKa' 8.72) ([51] ) and alphaprodine (pKa' 8.73) ([51] ) satisfy requirement ([1] ) in that they are substantially ionized as cations at physiological pH, and in both, the 4-phenyl substituent represents the necessary flat aromatic structure. Evidence of stereospecificity derived from study of analgesic antagonists is abundantly available in the case of pethidine-type compounds (references 52--59 relate to antagonism of pethidine by nalorphine, 54 and 55 to alpha-prodine by nalorphine, and 60 to alphaprodine by (-)-3-hydroxy-N-propargylmorphinan). Pethidine probably exists as an equilibrium mixture of the two conformational arrangements .(LXIV) and (LXV), the equatorial-phenyl form predominating owing to its greater thermodynamic stability (see fig. 1). Models reveal that both forms are capable of fitting the proposed receptor surface, although (LXIV) would be expected to fit more closely than (LXV), owing to its greater resemblance to the rigid morphine structure (XXVI). As the energy difference between the two forms is likely to be small, it is possible that the stability imparted upon absorption at the site, greatest in the case of the axial phenyl conformation, may override the non-bonded interaction factors in molecule itself in determining the conformation of pethidine at the site of action (similar arguments apply to the reversed esters of pethidine). The most probable conformations of alpha- and betaprodine have already been discussed (see "Stereochemistry" on p. 44). Models show that both isomers can fit the proposed receptor site, but of the two forms, the cis (Me/Ph) should fit better than the trans (Me/Ph) isomer. It is possible to explain the increase in activity of compounds obtained by the substitution of a 3-alkyl group into the reversed ester of pethidine in terms of a more bulky hydrocarbon moiety improving the fit in the cavity of the receptor site. In a recent paper, Beckett, Casy & Harper ([61] ) have advanced the hypothesis that oxidative dealkylation to produce nor-compounds at the "analgesic receptor site" is the first step in the reaction sequence leading to analgesia. The influence of the basic group upon analgesic activity may thus be considered from two aspects -- (1) the steric requirements of the anionic receptor site, and ([2] ) the ease of dealkylation. The activity of a series of methadone- and thiambutene-type analgesics has been shown to decrease upon increase in the "effective width" of the basic group, and from this evidence the anionic site of the receptor surface has been assigned certain dimensions ([62] ). The high activity of certain N-substituted norpethidine compounds must now be considered in the light of these facts. Evidence that the phenyl and benzyl groups are too large to fit at the anionic site is provided by the absence of activity in norpethidine-type compounds bearing these sub-stituents upon the nitrogen atom (1, 66). Increase of the chain length giving the N-phenylethyl compound results in an increase in activity over that of the parent compound. The steric limitations of the anionic site must therefore be less restricted farther removed linearly from the focus of charge, and may allow of additional van der Waals&rsquo force bonding between the aromatic ring at the end of the chain and the receptor site. The high activity of &beta-morpholinoethyl norpethidine and its sulphur analogue may be explained in a similar way, additional bonding being provided in these cases by the lone-pair electrons on the oxygen and sulphur atoms respectively (cf. the increased activity of the morpholino analogue of methadone over its parent compound ([63] )). The effect of chain branching upon activity may also be interpreted in terms of steric limitations at the anionic site. Branching at the carbon atom &alpha- to the piperidine ring nitrogen atom results in a greater reduction in activity than does branching at the &beta-carbon atom, where steric limitations are not so great. In addition to these steric factors it is also necessary to stress that the presence of electrical dipoles in the &beta-phenylethyl chain, etc., may result in an increase in the rate of dealkylation over that of pethidine leading to a consequent increase in activity. We wish to thank Wyeth Laboratories for making available information concerning the azacycloheptanes. We also thank the editor of the Journal of Pharmacy and Pharmacology for making available copies from which certain diagrams in the text were prepared. 14-carbethoxy-l-methyl-4-phenylpiperidine, also known as Meperidine and Dolantin. 2The corresponding N-tosyl compounds are also employed, conversion to the nor-compound (X) being carried out in this case by hydrolysis with sulphuric acid. ([24] ) 3If this isomer proves to be the one formed in major amount, it would be expected to have the trans (CH 3/C 6H 5) configuration from a consideration of the stereochemistry of addition to cyclic ketones (30). ReferencesBRAENDEN, EDDY & HALBACH, Bull. Wld. Hlth. Org ., 1955, 13, 937. 002CLARK, PESSOLANO, WEIJLARD & PFISTER III, J. Amer. chem. Soc ., 1953, 75, 4963. 003EDDY, personal communication cited by May. 7 004PERRINE & EDDY, J. org. Chem ., 1956, 21, 125. 005WEIJLARD, ORAHOVATS, SULLIVAN, PURDUE, HEATH & PFISTER III, J. Amer. chem. Soc ., 1956, 78, 2342. 006ORAHOVATS, LEHMAN & CHAPIN, J. Pharmacol ., 1957, 119, 26. 007MAY, J. org. Chem ., 1956, 21, 899. 008ANDERSON, FREARSON & STERN, J. chem. Soc ., 1956, 4088. 009MILLAR. & STEPHENSON, Brit. J. Pharmacol ., 1956, 11, 27. 010GREEN & WARD, ibid., 1956, 11, 32. 011JENSEN, LINDQUIST, PEKLING & WOLFFBRANDT, Dansk. Tidskr. Farm ., 1943, 17, 173. 012BERGER, ZIERING & LEE, J. org. Chem ., 1947, 12, 904. 013FOSTER & CARAMAN, J. Pharmacol ., 1947, 91, 195. 014RANDALL & LEHMANN, ibid., 1948, 93, 314. 015BACHRACH, GODHOLM & BETCHER, Surgery, 1955, 37, 440. 016GROSS, HOLLAND & SCHUELER, J. Applied Physiol ., 1948, 1, 298. 017GROSS, BROTMAN, NAGYBY, SAWTELLE & ZAGER, Fed. Proc ., 1949, 8, 297. 018HOUDE, RASMUSSEN & LA DUE, Ann. N.Y. Acad. Sci ., 1948, 51, 161. 019ISBELL, J. Pharmacol ., 1949, 97, 182. 020JANSSEN, private communication. 021McELVAIN & BARNETT, J. Amer. chem. Soc ., 1956, 78, 3140. 022HOWTON, J. org. Chem ., 1945, 10, 277. 023McELVAIN & STORK, J. Amer. chem. Soc ., 1946, 68, 1053. 024EISLEB, Ber. dtsch, chem. Ges ., 1941, 74B, 1433. 025SCHMIDLE & MANSFIELD, J. Amer. chem. Soc . 1955, 77, 5698. 026Idem., ibid ., 1957, 77, 5754. 027BECKETT & CASY, J. Pharm. Pharmacol ., 1954, 6, 986. 028BECKETT & WALKER, ibid., 1955, 7, 1039. 029ZIERING & LEE, J. org . Chem., 1947, 12, 911. 030BECKETT, CASY, KIRK & WALKER, J. Pharm. Pharmacol ., 1957, 9, 939. 031SEIFTER, ECKFELD, LETCHACK, GORE & GLASSMAN, Fed. Proc ., 1954, 13, 403. 032DIAMOND, Ph.D. thesis, 1955, Temple University, Utah. 033GITTINGER, GROSSMAN & BATTERMAN, Fed. Proc ., 1955, 14, 343. 034GOLBEY, GITTINGER. & BATTERMAN, ibid., 1955, 14, 344. 035GROSSMAN, GOLBEY, GITTINGER. & BETTERMAN, J. Amer. Geriatrics Soc ., 1956, 4, 187. 036BLICKE & TSAO, J. Amer. chem. Soc ., 1953, 75, 3999. 037SCHULTZ, ROBB & SPRAUGE, ibid., 1947, 69, 2454. 038NAZAROV & RUDENKO, Bull. Acad. Sci. U.S.S.R. Div. Chem. Sci ., 1948, 610. 039NAZAROV, SOKOLOV & RAKCHEEVA, ibid., 1954, 65. 040NAZAROV, RAIGORODSKAYA & RUDENKO, ibid., 1949, 504. 041GREBENNIK, Farmakol. i Toksikol ., 1954, 17, 48. 042NAZAROV, et al., Klin. Med. (Mosk) , 1952, 30, 60. 043NAZAROV, PROSTAKOV & SHVETSOV, Zhur. obschei Khim ., 1956, 26, 2798. 044MASHKOYSKII & ABRAMOVA, Farmakol. i Toksikol ., 1956, 19, 26. 045SCHAUMANN, Arch. exptl. Path. Pharmakol ., 1940, 196, 109. 046MACDONALD, WOOLFE, BERGEL, MORRISON & RINDERKNECHT, Brit. J. Pharmacol ., 1946, 1, 4. 047BECKETT, J. Pharm. Pharmacol ., 1952, 4, 425. 048BECKETT & CASY, Nature, Lond ., 1954, 173, 1231. 049BECKETT & CASY, J. chem. Soc ., 1955, 900. 050Idem, ibid ., 1957, 3076. 051FARMILO, OESTREICHER & LEVI, Bull. Narcotics , 1954, 6: 1, 7. 052RADOFF & HUGGINS, Proc. Soc. Exptl. Biol. N.Y ., 1951, 78, 879. 053SMITH, LEHMAN & GILFILLAN, Fed. Proc ., 1951, 10, 335. 054WINTER, ORAHOVATS, FLATAKER, LEHMAN & LEHMAN, J. Pharmacol ., 1954, 111, 152. 055GRUBER & GRUBER, ibid., 1953, 109, 157. 056ECKENHOFF, ELDER & KING, Amer. J. Med. Sci., 1952, 223, 491. 057BODMAN, Proc. Roy. Soc. Medicine , 1953, 46, 923. 058ECKENHOFF, HOFFMAN & FUNDBERG,. J . obst. Gyneo ., 1953, 65, 1269. 059ECKENHOFF, HOFFMAN & DRIPPS, Anaesthesiology, 1952, 13, 242. 060WHITE, MEGIRAN & MARCUS, Proc. Soc. Exptl. Biol ., 1956, 92, 512. 061BECKETT, CASY & HARPER, J. Pharm. Pharmacol ., 1956, 8, 874. 062BECKETT, CASY, HARPER. & PHILLIPS, ibid., 1956, 8, 860. 063DUPR?E, ELKS, HEMS, SPEYER & EVANS, J. chem. Soc ., 1949, 500. 064McELVAIN, DICKENSON & ATHEY, J. Amer. chem. Soc ., 1954, 76, 5625. 065SEIFTER & GLASSMAN, personal communication cited by Braenden et al. 1 066ELPERN, GARDNER & GRUMBACH, J. Amer. chem. Soc ., 1957, 79, 1951. 067SCHMIDLE & MANSFIELD, U.S. 2, 765, 315. 068DIAMOND, BRUCE & TYSON, J. org. Chem ., 1957, 22, 399. 069BENSON, CUNNINGHAM, HANE & VAN WINKLE, Arch. int. pharmacodyn ., 1957, 109, 171. 070MAY, J. org. Chem ., 1957, 22, 593. 071FRASER, Fed. Proc ., 1956, 15, 423. 072Bull. Narcotics, 1957, 9: 3, 27. 073ZIERING, MOTCHANE and LEE, J. org. Chem ., 1957, 22, 1521. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
