|
|
|
|
Bulletin on NarcoticsVolume LI, Nos. 1 and 2, 1999Occasional papers
Drug characterization/impurity profiling, with special focus on methamphetamine: recent work of the United Nations International Drug Control ProgrammeB. REMBERG A. H. STEAD Scientific Section, United Nations International Drug Control Programme, Vienna Abstract ABSTRACT In view of the increase in the manufacture of, trafficking in and abuse of synthetic drugs around the world, as well as the increasing involvement of organized criminal groups, there is an urgent need to identify the extent of such activity, the supply sources, the trafficking routes and the distribution patterns. Drug characterization/ impurity profiling are scientific tools used to support regular operational and intelligence work in this area by law enforcement authorities. Impurity profiling, the analysis of the various impurities in clandestinely manufactured drugs, by type and quantity, is not a routine technique. In order to provide more information on a seized drug sample than that obtained by using normal chemical analysis, and to identify any links between two samples of seized drugs, experienced chemists and dedicated equipment are required. In addition, close cooperation between forensic laboratories and law enforcement authorities is essential if all relevant information on links is to be followed up effectively. The present paper provides a brief introduction to drug characterization/and impurity profiling, focusing on activities and findings of the methamphetamine impurity profiling programme currently being carried out by the Scientific Section of the United Nations International Drug Control Programme.* IntroductionThe continuing demand for illicit drugs has led, in the last two decades, to the proliferation of clandestine laboratories synthesizing a wide variety of drugs of abuse in an increasing number of countries. Advances in chemical knowledge and technology, the increased availability of basic chemicals, precursors and equipment, and easier access to literature on the subject, including underground literature, have contributed to that development. As larger and larger consignments of clandestinely manufactured synthetic drugs are being intercepted in some regions of the world, law enforcement authorities require enhanced capacity to identify the supply sources of such drugs, the drug trafficking routes used, the distribution patterns followed and any links between samples of seized drugs. Drug characterization/impurity profiling, that is, the systematic characterization of seized drug samples by physical and chemical means, are valuable scientific tools used to support intelligence-gathering and operational work by law enforcement authorities. Throughout the world, characterization and impurity profiling of seized drugs are increasingly being used to complement routine investigative work by law enforcement authorities. Chemical links between samples may be established, material from different seizures may be classified into groups of related samples and the origin of samples may be identified. That information may be used for evidential purposes or it may be used as a source of more general intelligence to identify drug trafficking patterns and distribution networks. Drug characterization/ impurity profiling may also assist in identifying output from new illicit drug manufacturing laboratories and in monitoring methods commonly used in illicit drug manufacture, which, in turn, may provide information that is helpful to other intelligence-gathering units or regulatory authorities, for instance in programmes for monitoring precursors. Finally, drug characterization may provide supportive evidence in cases where there is a need to differentiate illicitly manufactured drugs from those diverted from licit sources. Recognizing the need for a cohesive international strategy in this area, the Commission on Narcotic Drugs, in its resolution 1 (XXXIX) of 24 April 1996, requested the Executive Director of the United Nations International Drug Control Programme (UNDCP) to develop standard methods for the profiling/signature analysis of key narcotic drugs and psychotropic substances. Since the adoption of that resolution, activities initiated by the Scientific Section of UNDCP have been aimed at developing methods for the characterization and impurity profiling of such substances, at supporting basic research to assist in the interpretation of analytical results and at contributing to the development of operational capacity, at the national and regional levels, in drug and precursor characterization and impurity profiling. Those activities also contribute to the development of greater intelligence capability, which in turn improves the capacity of UNDCP to estimate and forecast the drug problem. Pursuant to Commission on Narcotic Drugs resolution 1 (XXXIX) and the Action Plan against Illicit Manufacture, Trafficking and Abuse of Amphetamine-type Stimulants and Their Precursors, adopted by the General Assembly at its twentieth special session (Assembly resolution S-20/4 A of 10 June 1998), UNDCP activities have focused on methamphetamine and its main precursor, ephedrine, above all in south-east Asia, which has been particularly affected by the clandestine manufacture of, trafficking in, and abuse of that drug in recent years. The current involvement of UNDCP in drug characterization/impurity profiling is not new. Pursuant to Economic and Social Council resolutions 159 II C (VII) of 3 August 1948 and 246 F (IX) of 3 August 1949, an international programme for opium research was initiated as a result of concerns about the prevailing global situation with regard to the availability of opium and the multiplicity of source countries. The programme was aimed at developing methods for determining the origin of opium by chemical and physical means. The adoption by the Council of its resolution 548 D (XVIII) of 12 July 1954 led to the creation of the United Nations narcotics laboratory, the predecessor of the Scientific Section of UNDCP. A total of 148 research papers, published over a period of 16 years under the serial heading “The assay, characterization, composition and origin of opium”, summarize the results of that first comprehensive research programme on the subject carried out under the aegis of the United Nations. The development of methods for the characterization of opium was discontinued in the late 1960s, when efforts began to focus on heroin, which was being increasingly abused. Drug characterization/impurity profiling1For synthetic drugs, practical experience has shown that the impurity profiles of the products from a given illicit laboratory are characteristic. Provided that there is no change in the method or the conditions of drug synthesis, variations in the impurity content of drugs synthesized at different times by the same chemist in a clandestine laboratory are believed to be relatively small. Consequently, based on their chemical characteristics, samples of seized drugs can be classified into groups identified by their chemical impurity profiles, and a given sample or group of samples may be associated with an individual chemist or laboratory operating clandestinely. It is thus possible to link together illicit drug consignments from the same source and to build up a database of related drug samples over a period of time. Moreover, the presence of some characteristic cutting agents may indicate the involvement of certain illicit drug manufacturing or trafficking organizations. While such chemical profiling information may not help to identify the origin of a synthetic drug sample (that is, the location of the illicit drug laboratory), it may be used to link samples from a series of manufacturing batches2 to a single chemist or laboratory and to identify the source of supply or distribution. Similarly, starting materials used in illicit drug manufacture may also contain certain characteristic impurities. The impurity content and the type of impurities may vary depending on the nature of the starting material, on whether a precursor chemical was diverted from legitimate sources or was itself manufactured clandestinely. The identification of characteristic impurities (or impurity patterns) in precursors may therefore help to link them to a commercial or clandestine source. In addition, knowledge about the presence of certain impurities in starting materials may also help to link finished products to those starting materials, and ultimately to the source of such materials. Operational value to law enforcement investigationsFrom an investigative point of view, drug characterization may be carried out either for evidential purposes or for intelligence purposes: it may be used to help to confirm a connection between two or more samples in, for example, illicit drug supply cases for prosecution purposes; or it may be used to provide more general intelligence, for example, to identify local, regional or international distribution networks and sources of illicit drug supply, in support of law enforcement investigations. In particular, it may help:(a) to establish specific links between two or more samples of seized drugs; (b) to classify material from different drug seizures into groups of related samples, thus building up distribution networks; and (c) to identify the source of a sample of seized drugs. Such information may be used for evidential purposes or as a source of intelligence, to identify samples that have a common history. A fourth purpose of drug characterization is to monitor illicit drug manufacturing methods and the chemicals used. Thus, depending on the nature of the drug sample investigated, the information generated through drug characterization may be used to identify from where, how and to what extent a drug has been distributed. It may be used to provide intelligence on the number of sources of the drug (for example, on whether or not those sources are within a country) and on the drug distribution network (for example, on the points of distribution). Information obtained from drug characterization may also be used to estimate how long a particular clandestine laboratory has been operating and to assess the scale or output of an illicit drug operation. At the national and international levels, the examination of drug samples may provide valuable information on new or established drug trafficking routes and distribution patterns. The role of the drug analystIn recognizing the potential contribution of drug characterization/impurity profiling to the operational work of law enforcement authorities, it is important to recognize the role of the drug analyst. Irrespective of the equipment and software available in a laboratory carrying out the impurity profiling, the results have to be interpreted carefully by an experienced analyst, especially if they are to be presented in court. Several factors may complicate the establishment of links between drug samples. One such factor, independent of source or distribution, is the possibility of a change occurring in the impurity content of a drug sample as the main drug and/or its accompanying impurities decompose. Such a change (sometimes referred to as the “ageing” of a drug sample) may occur as a result of the conditions to which the sample is subjected, in particular exposure to light, heat and/or air. Thus, a chemical comparison of drug samples related by source yet exposed to different environments during the supply chain could suggest that the samples are not linked. In a similar way, certain aggressive cutting agents, such as ascorbic acid, may alter the composition of a drug sample over time; thus, a chemical comparison of older samples may not provide any useful information to help link the samples together. The analyst should take such considerations into account when interpreting and communicating the findings of drug characterization/impurity profiling to law enforcement authorities. Moreover, in order to draw correct conclusions from the comparison of chemical impurity profiles of different drug samples, it is necessary to assess the quality of the chemical link between the samples. The “strength of evidence” of results is determined by two factors: (a) the closeness of the correlation of two or more impurity profiles; and (b) the frequency of the particular profile pattern. An unusual profile pattern, that is, a profile that is not frequently encountered, or the presence of unusual individual components in the profile may increase the strength of evidence. For example, the presence of unusual cutting agents may provide a critical source of information to help link samples and may provide evidence of direct (immediate) links in conspiracy or dealer/user cases. The overall strength of evidence may be further improved by combining information from chemical profiling with information from other investigative approaches (such as information provided by examining the packaging materials). While, in some cases, visual (physical) examination of samples may provide additional evidence of a direct link between the samples, it is important to recognize that physical characteristics of tableted drugs (tablet markings (“logos”), shape, dimensions, weight) do not necessarily bear any relation to the location where the drug material (powder) was manufactured. Similarities in the physical characteristics of samples of seized tablets simply suggest a relationship at the level of the tableting laboratory; the drug powders themselves may be from different sources. Conversely, different physical characteristics do not necessarily mean that there is no relationship between the two samples; drug powders manufactured at the same time in the same laboratory may be tableted at different times using different moulds or different tableting machines. LimitationsThe key to successful programmes for drug characterization/and impurity profiling is cooperation, at all levels, and across borders. Considering the number of authorities, at the national and regional levels, involved in the control of illicit drug manufacture and trafficking, political commitment is essential. Frequently identified obstacles to increasing the use of programmes for drug characterization/impurity profiling at the operational level include the following: lack of authentic samples (i.e. samples where information on the source of manufacture or details of the synthesis route are available); lack of an appropriate mechanism, or even legislation, for the timely exchange of seized samples and relevant information; and a lack of understanding of the purpose, needs, possibilities and limitations of drug characterization/impurity profiling, resulting in a lack of collaboration and feedback between laboratories and law enforcement authorities. Critical areas from the point of view of the drug analyst include: (a) knowledge about clandestine manufacturing methods; (b) handling of samples and sampling for analysis; (c) procedures for the extraction and preparation of samples; (d) analytical procedure; (e) data evaluation; (f) use of intelligence; (g) training; and (h) international cooperation. Government agencies need to address all these areas when developing a programme using drug characterization/impurity profiling as operational tools. The role of the United Nations International Drug Control ProgrammeActivities of the Scientific Section of UNDCP in the area of drug characterization/impurity profiling are designed: (a)To encourage and assist target countries in their efforts to embark on impurity profiling activities, in order to enhance activities aimed at gathering operational intelligence related to trends in drug and precursor trafficking; (b)To promote the training of personnel involved in carrying out and utilizing impurity profiling, including law enforcement officers, intelligence and regulatory personnel, laboratory managers and analysts; (c)To encourage cooperation, at the local, regional and international levels, in the area of drug impurity profiling, including the development of a network of laboratories to collect and share information derived from the chemical analysis of seized drugs; (d)To develop and disseminate analytical methods for the characterization and impurity profiling of seized drugs and their precursors, to be applied at the national level, including manuals explaining the operational value; (e)To enable the Scientific Section to serve as a reference centre for methamphetamine characterization and impurity profiling. UNDCP will continue to carry out background work and make the findings of its work available, so that national laboratories with limited resources may build upon those findings. Specific activities include the collation of information on known manufacturing methods, the precursors and chemicals used, alternative precursors and chemicals, and the routes used for the synthesis of the precursors. Activities also include the collection, as well as the distribution to interested national laboratories, of the following: relevant articles from scientific literature; drug samples seized in different parts of the world; key starting materials from different sources; and reference samples of key impurities and analytical data on them. Finally, activities also include: experimental work and applied, scientific research on, for example, selected synthesis routes used in clandestine laboratories; research on the impurities of key starting materials; and research on the impact of certain cutting agents on impurity profiles. All of the above-mentioned activities are aimed at providing a practical basis that could be used as a starting point for meeting the requirements of individual countries that are establishing operational drug profiling programmes. UNDCP acts primarily as a facilitator and catalyst. All activities are carried out in close collaboration with scientists from national forensic laboratories already engaged in programmes in the area of drug characterization/impurity profiling. Clandestinely manufactured methamphetamine: manufacturing routes and impuritiesThe reasons for the presence of trace impurities in clandestinely manufactured drugs are manifold: impurities may be generated de novo, as by-products during drug manufacture; they may already be present in the starting materials, reagents and/or solvents and may be carried over unchanged to the final product; or they may arise from reactions of original impurities present in starting materials. One complication may be the presence of impurities that are unrelated to drug manufacture, such as inadvertent, external contamination, the deliberate addition of small quantities of certain substances to improve the “quality” or “saleability” of the end-product (for example, the addition of flavouring agents) and the introduction of impurities through contaminated cutting agents. In order to be able to assess the significance of the presence or absence of an impurity in the end-product, and to draw correct conclusions from similarities and differences in impurity profiles, it is crucial to understand the chemistry of illicit drug manufacturing processes, in particular, the generation and stability of impurities, the significance of individual impurities for a given synthesis route, and the extent of possible variation in impurity profiles of drugs synthesized via the same route. This information may be obtained by carrying out a detailed chemical analysis of samples of known manufacturing origin. In the absence of such authenticated samples, the link between synthesis routes and attendant impurities may be established through a series of controlled laboratory experiments on different synthesis routes used in clandestine drug manufacturing laboratories. Methamphetamine can be manufactured by a number of synthesis routes. Figure I shows the six most frequently encountered routes used in the clandestine manufacture of methamphetamine. Two major groups of synthesis can be distinguished: (a) syntheses starting from 1-phenyl-2-propanone (P-2-P) and yielding racemic methamphetamine, such as the Leuckart route and reductive amination; and (b) synthesis routes using optically purel-ephedrine or d-pseudoephedrine as starting materials, thus yielding the more potent d-methamphetamine. The latter include the Nagai route, Birch reduction, Rosenmund hydrogenation, and the Emde route with chloro-ephedrine as intermediate 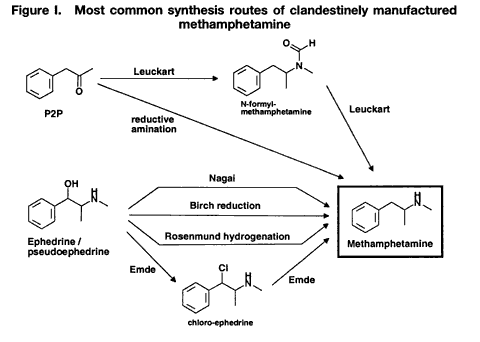 Only a few systematic scientific studies of samples of methamphetamine synthesized in controlled laboratory experiments have been published. There have been even fewer studies investigating how modification of the synthesis method may induce specific impurities, and the impact of small changes in the experimental conditions on the impurity pattern. Experience shows, however, that minor changes in synthesis can have a major impact on the formation of by-products, and thus on the complexity of the impurity profile. Parameters expected to have an impact on the impurity profile of the final product include: (a) the reaction temperature; (b) the reaction time; (c) the scale of reaction and the proportions of the initial chemicals; and (d) the extent and means of purification of intermediates and end-products [1]. It is difficult to duplicate clandestine manufacturing methods in laboratory studies. The reasons for this include: (a) the unavailability of authentic underground recipes;3 (b) the fact that the scale of reactions in controlled laboratory experiments is smaller than that used in clandestine laboratories; and (c) the need to take safety precautions in legitimate laboratories [2] (chemists working in clandestine laboratories normally do not take any such precautions and are not concerned about the environmental impact of their activities). In order to be able to assess the variability in impurity profiles generated during the synthesis of methamphetamine, UNDCP has carried out a limited number of laboratory experiments focusing on the most frequently encountered synthesis routes. Results from these “model syntheses” carried out under controlled laboratory conditions confirm a number of published findings [1, 2], and allow the following general conclusions to be drawn: (a)The relative similarity of the profiles obtained from a series of experiments using the same synthesis route may vary from one route to another; (b)There may be differences in the impurity profiles of products obtained from model syntheses carried out by using the same route but slightly modifying the recipes; (c)Even when samples are synthesized under controlled conditions in the same laboratory using the same recipe, batches with identical profiles are difficult to reproduce; (d)There are no significant differences in yield by most synthesis routes; (e)The impact of purification on the impurity profile depends on the synthesis route and varies from one impurity to another; (f)Purification of the crude base product by distillation significantly reduces the total intensity of the impurity profile; and the impact of purification of the hydrochloride end-product is also considerable and may even result in the removal of impurities, which are described in the literature as “route-specific”;4 (g)Re-use of the crystallization solvent (that is, the “mother” liquor) considerably increases the total profile intensity but does not have a significant impact on the pattern of the impurity profile. A review of the literature suggests the existence of certain “route-specific” impurities. Practical experience in the analysis of samples, however, shows that, for most synthesis routes, such “marker impurities” do not exist and there is some overlap between the impurities generated by different routes. Therefore, rather than individual “marker impurities”, it is the pattern of impurities and their intensity ratios in the impurity profile that seem to be characteristic of individual synthesis routes and different recipes. Methamphetamine characterization and impurity profiling: results and observationsChemical characteristics (impurity profiles)A selection of impurity profiles of seized methamphetamine is shown in figure II. 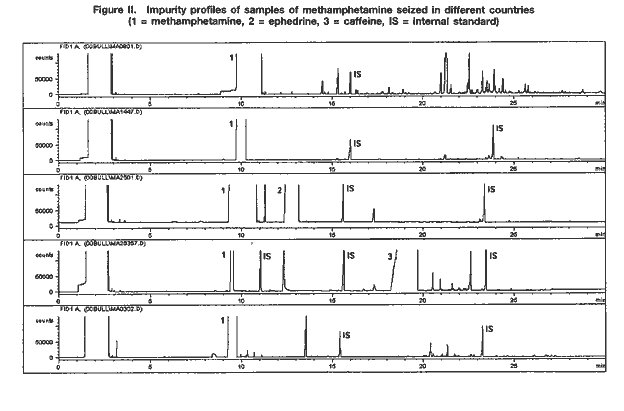 As can be seen, there are significant differences between impurity profiles. A comparison of the profiles of samples of seized methamphetamine with those from samples synthesized under controlled laboratory conditions revealed that the majority of samples examined appear to have been synthesized with ephedrine as the starting material. While samples from the Czech Republic and the United States of America seem to have been synthesized via different modifications of the Nagai route, some samples from east Asia and most samples from south-east Asia showed characteristics of the Emde route of synthesis (see figure III). In contrast, samples from Sweden are suspected to have been manufactured from P-2-P via the Leuckart route. 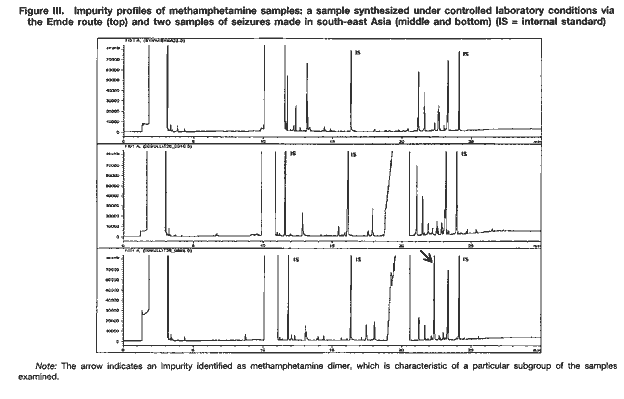 Because the work of UNDCP has focused on south-east Asia and because of the nature of samples from that subregion, detailed analytical investigations have concentrated on the Emde route. The distinctive feature in the impurity profile of methamphetamine synthesized via this route is a characteristic group of impurities at relatively high retention times (20-24 minutes) (see figure III). In terms of analytical findings, a number of those impurities have been characterized by their mass spectra and their molecular weights determined using gas chromatography-mass spectrometry with chemical ionization. None of the mass spectra have been found in the literature or in common mass spectral libraries. Amongst the samples of seized methamphetamine assumed to have been synthesized via the Emde route, differences in the impurity profiles were apparent. For example, a particular subgroup of samples was characterized by the presence of relatively large quantities of a substance identified as methamphetamine dimer (see figure III). Other subgroups could also be identified. The appearance of such distinct subgroups within samples may be attributed to modifications in the synthesis route. However, further work will be required to confirm this and to correlate specific reaction conditions with a particular pattern of impurities. Apart from synthetic impurities, the impurity profiles of some methamphetamine samples from south-east Asia also showed the presence of traces of N-acetylcodeine, 6-monoacetylmorphine (6-MAM) and, sometimes, codeine (see figure IV). This finding strongly supports the assumption that the illicit manufacture of methamphetamine and the refining of heroin sometimes took place at the same location (i.e. that illicit heroin manufacturers might be increasingly manufacturing synthetic stimulants as well). 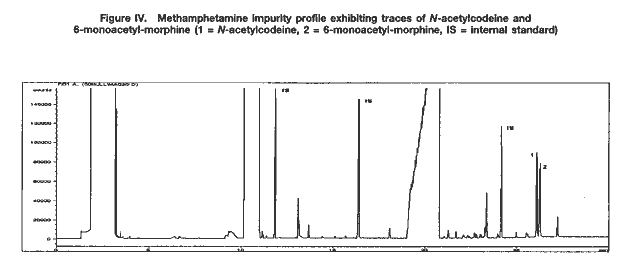 In terms of drug content, the typical (modal) content of methamphetamine hydrochloride in tablets from south-east Asia ranged between 25 mg and 30 mg. This converted to a purity of approximately 30 per cent relative to tablet weight. Caffeine was present in the majority of tablets, usually also in concentrations of approximately 30 per cent of tablet weight. In some samples, the presence of large amounts of ephedrine suggested that that substance, rather than representing unreacted starting material, had been added as an adulterant. The total content of extractable impurities (excluding caffeine and ephedrine) was usually less than 0.5 per cent. Physical characteristicsIn addition to findings from chemical impurity profiling, physical characterization of samples of seized drugs may provide valuable information in support of the operational work of law enforcement authorities. For synthetic drugs, this applies in particular to drugs in tablet form, where comparing, for example, their colours, logos, shapes, dimensions and weights can help to link them to a distribution network, a single source of production and, in some cases (by examining the defects or marks on the tablet surfaces), to the actual equipment used for tableting. A combination of the findings from both chemical and physical examinations is critical to the drawing of meaningful conclusions. Since the majority of the samples from south-east Asia were tablets, their physical characteristics were examined. They were found to differ widely. Although the UNDCP collection of tablets is far from comprehensive and other, more extensive collections exist, it was possible to make the observations presented below. In terms of logos, at least five types containing the letters “wy” were encountered among the tablets that were examined. Tablets with the two letters separated (not touching each other) and with an elongated letter “y” constituted the most common variant. Within that group, there were subgroups that could be identified by the size of the two letters, the length of the outer and inner lines of the letter “w”, the length and bend of the tail of the letter “y” and the width of individual lines of the impression. The first samples examined bearing the letters “wy”, currently by far the most frequently encountered logo on tablets from south-east Asia, were reported to have been from tablets seized in 1995. Samples of tablets seized before that date had a much larger variety of logos; the logo “99” was common.5 The typical weight of the tablets examined was between 80 mg and 95 mg (range: 60-115 mg); the typical dimensions were 6.0-6.1 mm in diameter and 2.9-3.2 mm in thickness. Nevertheless, a relatively wide range of figures was obtained for the overall weight, thickness and diameter, the latter, owing to the tableting process, being the least variable parameter. Various colours and shades were observed, but orange tablets seemed predominant in more recent samples. Examination of samples consisting of packs of 200 orange tablets with the logo “wy” and one or two green tablets inserted as markers6 revealed that the impurity profiles of the two tablet types were not necessarily the same (see figure V). That finding suggests that the methamphetamine powders used to prepare the orange and green tablets were manufactured at different times. 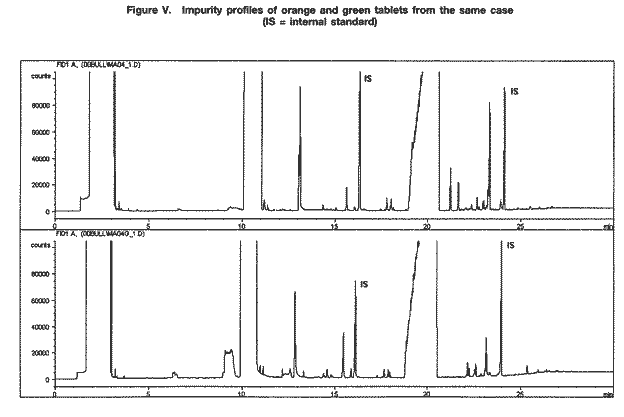
Chemical and physical characteristics combinedJust as the orange and green tablets referred to above were shown to have different impurity profiles, samples of tablets with the same physical characteristics that have been seized in the same location may have different profiles. Conversely, similarities between physical characteristics of tablets have been confirmed by UNDCP impurity profiling work, even though the tablets had been seized in different locations (see figure VI). 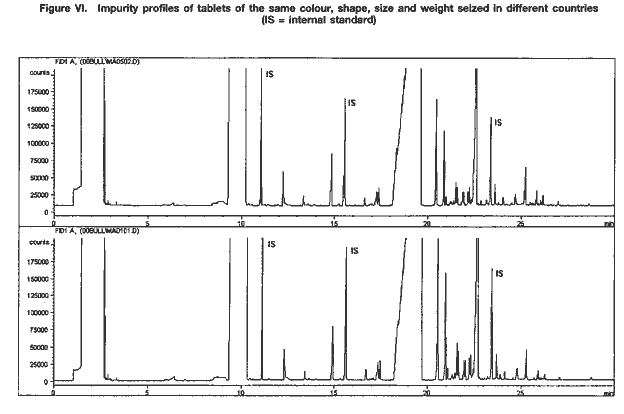 The case described below illustrates the complexity of the links between physical and chemical information obtained from drug characterization/impurity profiling studies. In addition to providing direct support to law enforcement authorities working on an individual case, the results of such studies, if complemented with other information, can also provide intelligence that may be useful in understanding patterns of illicit methamphetamine manufacture and trafficking in a region. In May 1999, a number of methamphetamine samples were received through the International Criminal Police Organization (Interpol). Although the samples were reported to have been seized in the same location, an examination of the background information seemed to indicate that they might have been seized in different locations, though in close proximity to each other. The samples consisted mainly of broken, presumably granulated tablets, and of tablets of inferior quality. They were packed in several plastic bags. While the contents of the bags were clearly distinguishable from each other by colour, three of the bags contained similar, homogeneous, broken material. Another bag contained partly intact tablets of inferior quality, whose physical characteristics (colour, shade, thickness, logo) differed. In addition, 10 dark orange, intact tablets of higher quality (based on physical appearance) were received. There were also two other samples: one consisted of an orange powder reported to be dyeing powder; the other consisted of a white, fluffy powder with a strong, sweet smell, reported to be ephedrine. The impurity profiles of all the methamphetamine samples showed characteristics of methamphetamine manufactured via the Emde route of synthesis. Two subgroups of samples could be distinguished: one with intact and partly intact tablets and the other with ground tablet material. All the samples contained traces of ethyl vanillin7 and relatively large amounts of N-methylephedrine. Caffeine was present as an adulterant. The purity of the broken or granulated tablet material was less than 20 per cent, while the intact tablets contained between 20 mg and 30 mg of methamphetamine hydrochloride (converting to a purity of 30 per cent relative to tablet weight). The 10 dark orange tablets were of the highest quality, in terms of both their physical characteristics and chemical impurity profiles. With the exception of one type of tablet of inferior quality, which did not bear any logo, the logo of the intact tablets was “wy”. The “wy” imprint on the dark orange, high-quality tablets differed from that on the other tablets seized. Some samples, although physically similar to the others, contained caffeine, ephedrine, N-methylephedrine and, in one case, also ethyl vanillin but little or no methamphetamine (less than 1 per cent). The powder reported to be dyeing powder was found to be similar, in terms of its impurity profile and overall purity, to the broken or granulated tablet material; thus, it seems to have been a homogeneous mixture of dyeing powder, methamphetamine, caffeine and ephedrine, ready to be tableted. Finally, the white, fluffy powder reported to be ephedrine was identified as pure ethyl vanillin. The following information, as an example, may provide useful intelligence: (a)Different types of methamphetamine were identified, indicating that the conditions of synthesis had been modified; this suggested that the seized methamphetamine might have been manufactured in more than one batch and/or in different locations; (b)Tablets of good and poor quality were seized, as well as tablets with different or no logos, suggesting that different tableting specialists and equipment had been used and/or that the tableting had been done in different locations; (c)Relatively large amounts of N-methylephedrine were present in the samples examined, indicating that that substance may have been used in the synthesis, either intentionally, as a result of a shortage of ephedrine, or unintentionally, having been presented and sold as “ephedrine”. In addition: (a)Similar sample material may have been manufactured in different locations using the same synthesis route, underlining the potential of drug characterization/impurity profiling to provide supportive evidence for law enforcement operations; (b)All samples contained, to a greater or lesser extent, ethyl vanillin and N-methylephedrine, impurities not frequently encountered, thus increasing the strength of evidence of a link between the different samples; (c)The seized material included several bags of broken or granulated tablets and tablets of inferior quality, suggesting the existence of some kind of quality control procedure, whereby tablets of inferior quality could be withheld from the illicit market; (d)Seized sample material was mislabelled or incorrectly reported, highlighting the need for appropriate training of personnel and for close collaboration between law enforcement and laboratory staff. Development of an analytical procedureAll the findings presented above were generated using an analytical method developed by UNDCP. In 1997, when work on developing the method started, there was no suitable method available for the impurity profiling of methamphetamine tablets encountered in south-east Asia. Methamphetamine is chemically related to amphetamine, on which there is a vast body of literature; however, because of distinct features of the methamphetamine encountered in south-east Asia, the methods described in the literature could not be easily applied. The factors complicating the impurity profiling of the methamphetamine samples included the following: the low level of the impurities present; the nature of the impurities (almost exclusively basic impurities in methamphetamine vs. some neutral key impurities in amphetamine); the wide concentration range (from parts per million to percentage levels) of impurities encountered in the methamphetamine samples; the presence of tableting aids, adulterants and diluents, interfering with the extraction of the impurities; and the presence of ephedrine in amounts that suggested that that substance, rather than representing unreacted starting material, had been used as a diluent. Based on samples of seized methamphetamine from 17 countries throughout the world, a method for the extraction of samples and their gas chromatographic analysis was developed. The method was optimized with regard to the extracting solvent, the pH for extraction, the amount of sample required and the analytical parameters, in particular the temperature programme used for gas chromatography. It was then refined and evaluated, to the extent possible (for instance, experiments on repeatability and long-term reproducibility were carried out), with emphasis on the type of methamphetamine currently encountered in south-east Asia. More than 500 samples, mainly from Thailand, but also from the Lao People’s Democratic Republic, Myanmar and Viet Nam, were used. The method was also applied successfully to drug powders and crystalline samples8 from other parts of the world, Australia, the United States and countries in east Asia and Europe (see figure II). The following common guiding principles were used to develop the method: (a)The sample pretreatment (extraction) procedure should be as simple as possible; (b)The analytical procedure should ensure optimal peak resolution; (c)The entire methodology should be robust and reproducible over a long period; (d)It should be possible to search and compare the resulting analytical data in a reliable and rapid manner. The parameters of the method developed are described below. Sample extractionA total of 30 mg of homogenized methamphetamine was dissolved in 1 ml of pH 10.5 buffer solution (pH 7 phosphate buffer + 10% Na2CO3 = 4+1 (v/v)) by shaking for 5 minutes. The solution was extracted with 0.2 ml of ethyl acetate (containing n-tridecane at 25 mg/l, diphenylamine at 35 mg/l and n-tetracosane at 20 mg/l as internal standards) by shaking for 5 minutes. It was then centrifuged for 5 minutes at 3,000 rpm and the organic layer was transferred to a small autosampler vial. The sample was analysed on the day of extraction. ApparatusA gas chromatographic system equipped with a flame ionization detector (GC-FID) was used (HP 5890 Series II GC, equipped with HP 7673 Injector). The parameters were as follows: Column: 25 m x 0.2 mm x 0.33 µm Ultra-2 (HP) Injection volume: 1 µl, splitless (1 min) Carrier gas: nitrogen (150 kPa = 1.4 ml/min at 50° C oven temperature) Temperature programme: 50 (1 min), 10° C/min to 300° C (4 min) Injector/detector temperature: 250° C/300° C FID gases: air: 2.5 bar (flow rate: approximately 300 ml/min) hydrogen: 1.4 bar (flow rate: approximately 30 ml/min) Septum purge: 3 ml/min Split vent: 30 ml/min Data system: HPChemStation, Revision A.05.03 [273], Hewlett Packard Signal parameters: Peak width: 0.013 Sampling rate: 10 Hz Signal plot: 10% offset ConclusionsUNDCP has in recent years been engaged in the development of standard methods for the profiling/signature analysis of key narcotic drugs and psychotropic substances. So far, work has concentrated on methamphetamine and its main precursor ephedrine. Methods have been developed for the characterization and impurity profiling of those substances, and basic research has been undertaken to assist in the interpretation of analytical results. The work has focused on south-east Asia. Analysis of samples using the new impurity profiling method has enabled the identification and/or confirmation of new trends in illicit methamphetamine manufacture and the development of operational intelligence by law enforcement authorities in the countries concerned. As a result, more and more countries have shown an interest in the potential of drug characterization/impurity profiling, highlighting the need for regional and international cooperation. UNDCP has encouraged such cooperation through, for example, closer liaison with Interpol. It has also assisted target countries in their efforts to embark on profiling activities and served as a reference centre for methamphetamine profiling. It should be recognized that drug characterization is a multidisciplinary activity whose usefulness can be maximized if close collaboration between laboratory personnel, police and customs authorities and an understanding of its purpose, needs, possibilities and limitations are ensured. Since the specific aim of any comparative study determines the analytical approach, law enforcement authorities must clearly specify the information that they expect from the forensic scientist. It should be also recognized that drug impurity profiling is not a routine analytical technique. In order to provide more information on a seized drug sample than that obtained by using normal chemical analysis and to identify any links between two or more samples of seized drugs, experienced chemists and dedicated equipment are required. Moreover, any programme for drug characterization/impurity profiling must be ongoing to ensure that databases of results are properly maintained and remain useful. In the future, in addition to developing analytical methods for the characterization and impurity profiling of various drugs of abuse, UNDCP will help to develop and strengthen operational programmes at the national and regional levels. To that end, it will develop guidelines for introducing analytical methods into operational programmes and will continue to analyse selected samples from key countries. Results will be shared with law enforcement officials and drug analysts in the countries concerned: (a) to raise awareness of the goals and limitations of drug characterization/impurity profiling in support of law enforcement activities aimed at gathering drug intelligence; (b) to show the potential of profiling in the investigation of illicit drug manufacturing routes and, ultimately, in programmes for monitoring precursors; and (c) to advance the area of law enforcement investigation and improve collaboration between law enforcement officials and forensic chemists. The value of such a role for UNDCP and the unique capacity of UNDCP to coordinate and facilitate cooperation in the development of national drug characterization/impurity profiling programmes have already been confirmed by countries in south-east Asia faced with the growing problem of illicit manufacture of and trafficking in methamphetamine, some of which have already started to develop their own operational drug profiling programmes. References
FOOTNOTES*Much of the work undertaken as part of the methamphetamine impurity profiling programme has been supported financially by the Government of Japan. The authors wish to acknowledge the technical assistance of B. Reiter in sample analysis under the impurity profiling programme. 1In a manual available from UNDCP, entitled Drug Characterization/Impurity Profiling: Background and Concepts (ST/NAR/32), more detailed information is provided on the characterization and impurity profiling of both plant-based and synthetic drugs. 2Drugs manufactured in a single synthesis (that is, in the same process) are referred to as a “batch”. 3“Synthesis route”, “synthesis method” (“recipe”, “modification”) and “batch” are used in the following manner: “synthesis routes” are the major ways of synthesizing methamphetamine (Leuckart route, Nagai route, Emde route etc.); a “synthesis method” is a particular modification of a synthesis route that follows a specific “recipe” describing the ratios of reagents and details of the reaction conditions (temperature, duration of the reaction etc.); and drugs manufactured in a single synthesis, that is, in the same process, are referred to as a “batch”. 4“Route-specific” means unique to a single synthesis route. 5In order to be able to analyse trends for intelligence purposes, it is essential to establish and maintain databases. The collected information could be more widely disseminated, for example, in the form of charts displaying logos appearing on methamphetamine tablets encountered, as has been done in a number of countries worldwide. 6The practice of adding one or two green tablets to a pack of 200 orange tablets, possibly as part of some sort of quality control procedure, is confirmed by law enforcement authorities in the subregion. 7Ethyl vanillin is used for legitimate purposes as a flavouring agent and in perfumery. It was later confirmed that, in the region in question, ethyl vanillin was sometimes added to clandestinely manufactured methamphetamine tablets because of its strong vanilla-like odour and taste. By mid-2000, many samples believed to have originated in the region were shown to contain ethyl vanillin. 8Analytical difficulties have been encountered with a certain type of methamphetamine of very high (>98-99%) purity (commonly called “ice”) from east Asia.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|