Contents
- Introduction
- What is Distillation?
- States of Aggregation
- Boiling Point
- Dependence of Boiling Temperature on Pressure
- Heat of Vaporization
- Heat Transmission During Boiling and Condensation
- Boiling of Liquids
- Bumping
- Separation of Mixtures
- Definitions
1. Introduction
Remember the first time you ever boiled water? You will certainly have noticed how the steam formed running droplets, i.e. condensed, on nearby surfaces. This was your first distillation. In the following report you will learn how to carry out distillations along more scientific lines.
2. What is distillation?
Distillation is one of the oldest methods of separating liquid or molten substances. During distillation the particles of a liquid are evaporated by the input of external heat. These gaseous particles are then condensed by cooling, i.e. they are transformed from the gaseous state back to their liquid state.
During this process only one phase moves, i.e. the vapor of the boiling liquid.
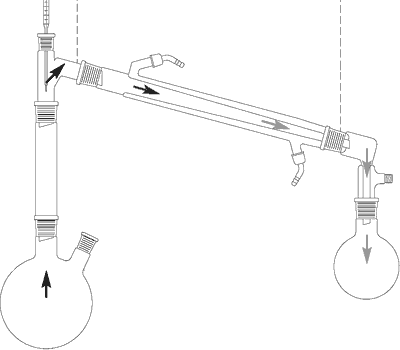
Purpose of distillation
Distillation is a fast, simple and effective method of cleaning and separation. It separates solvents and volatile substances from non-volatile liquids and solids.
(Solvents = e.g. water, alcohol, ether, petrol, chloroform, acetone, etc.)
Applications are:
- Concentration of solutions
- The solvent is distilled, leaving behind
a higher-boiling or solid residue. - Cleaning of volatile substances
- If a volatile product is contaminated with colored, resinous or high-boiling components, these contaminants remaining in the evaporating flask while the cleaned substance condenses in the condenser and is collected in the receiving flask.
3. States of aggregation
 | Solid |
| A substance is solid when the kinetic energy of the particles is less then their mutual attraction | |
 | Liquid |
| A substance is liquid when the kinetic energy of the particles is great enough to balance their mutual attraction. The particles are able to move among themselves but without separating. | |
 | Gaseous |
| A substance is gaseous when the kinetic energy of the particles is so great as to overcome their mutual attraction. The particles are able to move freely. |
The smallest particles (molecules, atoms, ions) of a substance are held together more or less strongly by mutual attraction (cohesion). A substance's state of aggregation, i.e. its physical condition, depends by and large on the intensity of this attraction and on the kinetic energy of the particles.
There are three characteristics states of aggregation (physical conditions)
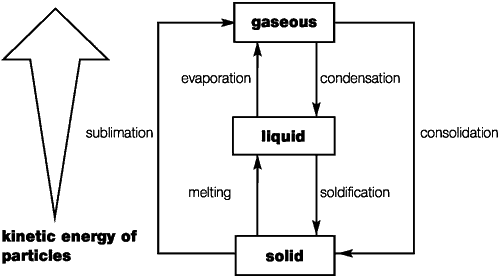
Changes of Physical
Conditions
If the state of aggregation changes from solid to liquid, we talk of melting; in the reverse case we speak of solidification. Changing from the liquid to gaseous state is called evaporation or boiling; the reverse is known as condensation or liquefaction.
Direct changes of phase from solid to gaseous and vice versa are also possible. In both cases we speak of sublimation, which literally means suppression (of the liquid state).
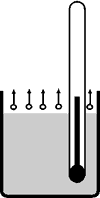
4. Boiling point
 |  | Heating Bath |
Evaporation |
Ebullition |
The boiling point of a liquid is that temperature at which its vapor pressure equals the ambient pressure and at which the change-over from liquid to gaseous state takes place. A liquid boils when it reaches its boiling point.
The boiling point is a substance-specific physical variable (e.g. 100°C for water, 78°C for ethanol). As a rule, a liquid can only be heated up to its boiling point (see "9. Bumping" for exceptions).
Evaporation/ebullition
There are big differences between the evaporation and ebullition of a liquid.
- Evaporation
- Molecules evaporate only at the surface of the liquid. The necessary energy is drawn from the surroundings. The temperature of the liquid drops.
- Ebullition
- Molecules evaporate throughout the liquid. The necessary energy needs to be input by heating.
- Vaporization
- Vaporization generally means a change from liquid to gaseous state at higher temperatures. The term has not yet been clearly defined.
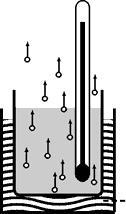
 |  |
Low Vapor pressure |
High Vapor Pressure |
Vapor Pressure
Molecules or particles which dissociate from the surface of a liquid exert a pressure against their surroundings; this is known as the liquid's vapor pressure.
A liquid's vapor pressure depends solely on the liquid's temperature and is a measure of a compound's volatility.
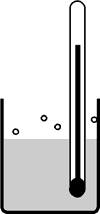
5. Influence of pressure on boiling point (atm. press.)
 |  | Air molecules Solvent or substance molecules |
Normal Pressure | Vacuum | |
Dissociation of liquid particles at atmospheric pressure. Struggle against many air molecules. | Dissociation of liquid particles at reduced pressure (vacuum). Struggle against fewer air molecules. | |
High Boiling Point | Low Boiling Point |
Contrary to the melting point, the temperature of ebullition depends greatly on pressure. The higher the ambient pressure, the higher the temperature of ebullition; the lower the ambient pressure, the lower the temperature of ebullition (e.g. water: 100°C in Nice at sea level, but approx. 84°C on Mont Blanc at 4810 meters altitude).
High-boiling substances can be distilled at a lower boiling temperature if their ambient pressure is reduced.
In practice, distillations are carried out at reduced pressure (vacuum distillation) in order to prevent damage to temperature-sensitive substances. Substances with a boiling point of 100°C or higher are often distilled in vacuum in order to use a water bath as heat source (temperature range up to max. 100°C).
6. Heat of vaporization
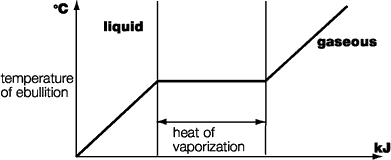
When a liquid reaches the temperature of ebullition, it evaporates without any further rise of temperature.
During evaporation the liquid needs to receive a continuous supply of energy in the form of heat to overcome the forces of molecular attraction. This heat supply is called the heat of vaporization.
e.g. Water |
e.g. Dioxane | ||
 | |||
335 kJ (80 kcal) heating to boiling point from 20°C to 100°C | 2261 kJ (541 kcal) evaporation at 100°C | 167 kJ (40 kcal) heating to boiling point from 20°C to 101°C | 360 kJ (86 kcal) evaporation at 101°C |
The heat of vaporization is a substance-specific physical variable. Contrary to the boiling point, however, it is practically independent of pressure. The specific heat of vaporization is the amount of heat (in kJ) required for 1 kg of the liquid to evaporate. The unit is expressed in kJ/kg. A substance's heat of vaporization is the same regardless of whether the liquid passes into the gaseous state as the result of boiling or pure evaporation.
Substance |
Boiling Point |
Heat of Vaporization |
|
kJ/kg |
(kcal/kg) |
||
| Xylene | 144°C | 345 | (82) |
| Toluene | 110°C | 347 | (83) |
| Dioxane | 101°C | 360 | 86) |
| Water | 100°C | 2261 | (541) |
| Ethanol | 78°C | 837 | (200) |
| Chloroform | 62°C | 246 | (59) |
| Aceton | 56°C | 546 | (131) |
The heat of vaporization is independent of the boiling point, i.e. a high boiling point does not automatically mean a high level of vaporization heat, as the following examples indicate:
Water has the highest heat of vaporization of all common solvents. All principles concerning evaporation and the heat of vaporization apply in reverse for the condensation and the heat of condensation.
7. Heat transmission during boiling and condensation
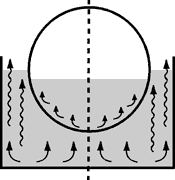 |  |
Convection |
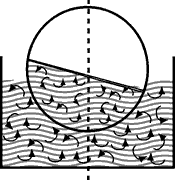
Turbulence |
The hot liquid expands, becomes lighter as the result and rises, while the colder layer sinks slowly to the bottom. Movement - and hence temperature balancing - takes place very slowly |
Layers of the liquid are mixed mechanically by stirring (or rotation of the evaporating flask in the water bath). Mixing - and hence temperature balancing - takes place quickly. |
This process is also called Free Convection | This process is also called Forced Convection |
When a vessel is heated from below, the vessel wall becomes hot first and then passes on the heat to the vessel contents. The heated liquid (e.g. water) rises and the colder liquid sinks to the bottom of the vessel. This is called convection.
Heat transmission by convection proceeds very slowly and is dependent on such physical variables as viscosity, density, thermal conductivity and thermal coefficients of expansion. If the liquid is moved and mixed in the heating bath by some additional means, e.g. by using a rotating flask, we speak of "forced convection".
Heat transmission
Those variables linked with the phase transition (heat of vaporization, boiling point, surface tension) are not important until boiling commences.
The energy required on the evaporator side can be supplied selectively and in concentrated form, but when it comes to removing this energy on the condensation side it has to be "collected" from the widely distributed gas particles. Hence the condensing area is generally several times bigger than the heating area.
8. Boiling of liquids
Vapor bubbles |  | 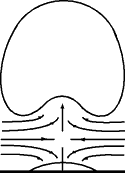 |
 | ||
Nucleation: Fine pores and irregularities in the vessel surface which trap fine air bubbles during filling. |
Breaking away: As a rule, there is no clean separation of the bubble from the vessel wall, leaving residual vapor. |
Liquid: Flowing of the partial conden- sation of the residual vapor, formation of new nucleations at the same point. |
On the walls and the bottom of a vessel there are fine pores and irregularities which trap very small air bubbles when liquid is filled in. These extremely fine bubbles are enlarged by thermal expansion.
Evaporating particles of liquid pass into these bubbles, causing them to grow until they finally break away and rise as boiling bubbles. This results in the familiar appearance of a boiling liquid → nucleate boiling.
Nucleate boiling
If there are no such pores or irregularities and hence no fine air bubbles to act as nucleations, they are created artificially, e.g. with boiling stones, or the hot liquid is moved to the surface by some additional means (e.g. stirring, rotation of the evaporating flask, etc.).
9. Bumping
If there is no nucleation in the form of air bubbles and if the liquid is not moved by some additional means, it cannot evaporate. The liquid overheats.
The convection current is very slow and is unable to move and distribute the input heat quickly enough. Evaporation starts abruptly when the extremely overheated liquid finally reach the surface as the result of the slow convection.
The escaping vapor bubbles can carry over a large part of the liquid. Instead of small vapor bubbles rising to the surface of the liquid one at a time, they join together at the bottom of the vessel to form large vapor bubbles which then rise suddenly to the surface with a powerful thrust. This phenomenon is often seen in liquids with sediment.
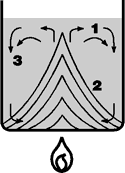 |
The convection current is very slow and unable to transfer the input |
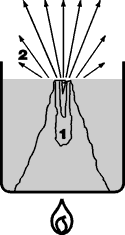
|
|
Bumping can be prevented by stirring or shaking the liquid, or by using boiling stones.
10. Solids and high-boiling solvent residues
Separation of mixtures
If there is a difference of at least 100°C between the boiling points of the substances to be separated, separation is possible with little effort:
- When distilling a mixture of solvent and solids, the solvent evaporates while the solids remain as residue.
- The procedure is the same for separating solvent from high-boiling residues (e.g. oils, fats, glycerine, etc.)
Complete separation is possible with a single distillation run.
Low-boiling residues
If there is only a small difference between the boiling points of the substances to be separated, a single evaporation and condensation run is unable to separate the substances with sufficient purity; the process has to be repeated several times.
Technical distillation systems are designed to permit multiple-stage distillation in continuous mode. In this case we no longer speak of distillation but of rectification or fractional distillation.
11. Definitions
- Volatility
- Tendency of a liquid to pass into the gaseous state without any great input of heat.
- Substance-specific, physical variable
- Each substance has its own characteristics such as melting point, boiling point, etc.
- Viscosity
- The resistance of a liquid to flow (e.g. honey).