
Filtration is a technique used either to remove impurities from an organic solution or to isolate an organic solid. The two types of filtration commonly used in organic chemistry laboratories are gravity filtration and vacuum or suction filtration.
Gravity filtration is the method of choice to remove solid impurities from an organic liquid. The "impurity" can be a drying agent or an undesired side product or leftover reactant. Gravity filtration can be used to collect solid product, although generally vacuum filtration is used for this purpose because it is faster.
A filtration procedure called "hot gravity filtration" is used to separate insoluble impurities from a hot solution. Hot filtrations require fluted filter paper and careful attention to the procedure to keep the apparatus warm but covered so that solvent does not evaporate. Hot gravity filtrations are no longer included in the routine procedures for the experiments in the organic chemistry teaching labs, they used to be used in the teaching labs to remove powdered Norite from a hot solution; since we switched to pelletized Norite, hot filtrations are not used. If you need to do such a filtration, read the procedure in the Handbook and consult your TA.
Procedure for standard gravity filtration
1) Select and fold the filter paper
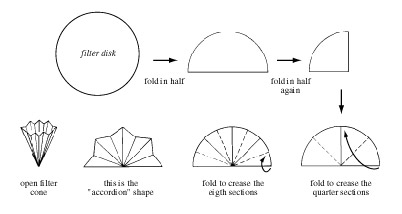
Select the size of filter paper that, when folded, will be a few millimeters below the rim of your glass funnel. Fold the paper into a cone by first folding it in half, and then in half again, as shown.
 |
 |
2) Filter the solution
Support the glass funnel in a ring or place it in the neck of an Erlenmeyer flask. Wet the filter paper with a few milliliters of the solvent to be used in the following procedure. Wetting the paper holds it in place against the glass funnel. Pour the mixture to be filtered through the funnel, in portions if necessary.
 |
 |
Note: fluted filter paper is often better for gravity filtration with organic solvents (see page 106 of the Handbook).
 |
||||
| diagram of how to fold fluted filter paper |
Vacuum filtration is used primarily to collect a desired solid, for instance, the collection of crystals in a recrystallization procedure. Vacuum filtration uses either a Buchner or a Hirsch funnel.Vacuum filtration is faster than gravity filtration, because the solvent or solution and air is forced through the filter paper by the application of reduced pressure. The reduced pressure requires that they be carried out in special equipment:
| Note: Do not use vacuum filtration to filter a solid from a liquid if it is the liquid that you want and if the liquid is low boiling. Any solvent which boils at about 125 degrees or lower will boil off under the reduced pressure in the vacuum flask. |
Procedure for vacuum filtration
1) Assemble the apparatus
Check the side arm flask (filtering flask or vacuum flask) carefully for cracks, since cracks could cause the flask to break when vacuum is applied. Then, clamp the flask securely to a ring stand. Add an adaptor and a Buchner funnel. Place a piece of filter paper in the funnel that is small enough to remain flat but large enough to cover all of the holes in the filter.
 |
 |
 |
|||||||||
|
|
|
|
|||||||||
 |
 |
 |
|||||||||
|
|
|
|
|||||||||
 |
|||||||||||
| Note on Vacuum Sources: Use the mechanical vacuum systems whenever possible. See the vacuum system page for a discussion of these systems and water aspirators.
If you use a water aspirator, read on: The photo to the left shows the vacuum filtration glassware connected to a "water-trap". The black tubing on the water-trap would be connected to a water aspirator. Whenever you use a water aspirator, you run the risk of sucking water from the aspirator into your vacuum filtration unit. The water-trap placed in-line will catch any such water. If you do NOT need the filtrate, but only need the solid matter collected on the Buchner funnel, this presents no problem. Most of the time this is the case, and usually students do not need a water-trap. If you do need one, they are located under the main hood or on the back shelves of each lab room. You never need a water-trap when using the mechanical vacuum systems. |
|||||||||||
2) Wet the paper with a small amount of the solvent to be used in the filtration. Turn on the vacuum source.
 |
||||
| Wet the paper - this causes the paper to adhere to the plate and keeps materials from passing under the paper during filtration.
Make sure that the paper is secure on the filter, that air is being drawn through the paper, and that all of your apparatus is securely clamped. If you are using a Neoprene filter adaptor, you might need to press on the funnel to engage the seal and thus the vacuum. Now you are ready to begin filtration. |
||||
3) Filter the solution
Pour the mixture to be filtered onto the filter paper. The vacuum should rapidly pull the liquid through the funnel. Watch that particulates do not creep under the edges of the paper. If this happens, start over and carefully pour portions of the solution onto the very center of the paper.
 |
 |
|||||
| Watch that particulates do not creep under the edges of the paper. If this happens, start over and carefully pour portions of the solution onto the very center of the paper. | Notice that the vacuum has pulled the solvent through the filter and into the filter flask. | |||||
4) Rinse the solids.
Rinse the cake with a small amount of fresh, cold solvent to help remove impurities that were dissolved in the filtrate. Disconnect the rubber tubing before turning off the water aspirator. Remove the filter paper and the collected solid that is on it. Usually you will set it on a watch glass and let it air dry for a while.
 |
 |
|||||||||
| Rinse the flask with a little fresh solvent and pour it into the filter funnel. | Disconnect the vacuum AT THE FLASK and before turning off the water aspirator. This prevents water from being sucked into the vacuum flask. | |||||||||
 |
 |
|||||||||
| Carefully remove the filter paper and solid from the Buchner funnel. | Set the filter cake onto a watch glass to air dry. | |||||||||
![]()