Abstract
Methaqualone (2-methyl-3-o-tolyl-4(3H)-quinazolinone) is the illicit synthetic drug of choice amongst South African drug users. Historically police and forensic investigation has proven that all methaqualone seized by the South African Police Service originates from illicit manufacturing sites both inside, and outside South Africas borders. From a drug enforcement, and forensic point of view it is, thus, of utmost importance that the various synthetic routes available to the illicit "chemist" are fully documented and understood. This is a prerequisite for effective illicit laboratory investigation, as well as chemical and precursor monitoring. This paper gives a brief introduction to the current status with regard to methaqualone use and production in South Africa, as well as an extensive review of the synthesis of methaqualone and selected isomers reported since 1946. A table summarizing synthetic routes reported in 32 reference sources is provided.
1. Introduction
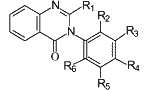
Fig. 1.
General structure of 2-alkyl-
3-aryl-4(3H)-quinazolinones.
The synthesis of methaqualone (I, Fig. 1, R1= Me, R2 or R6 = Me, R3=R4=R5=H) was first reported in 19511. It was introduced pharmaceutically as a non barbiturate, nonaddictive "sleeping pills" in 19652. It has been listed in the US Federal Register of March 1966 as an approved sedative-hypnotic with trade name Quaalude3. The abuse potential of methaqualone quickly became apparent resulting in it being listed in the 1971 United Nations (UN) Convention on Psychotropic Substances, and its subsequent banning in most member countries4. Methaqualone is currently listed in the UN Convention on Psychotropic Substances of 1988.
The production, trafficking, and abuse of methaqualone are of particular forensic importance to South Africa as it remains the synthetic drug of choice amongst South African drug abusers5,6. This is illustrated by the fact that methaqualone-seizures amounts to more than 60% of all street-drug seizures submitted to the South African Police Services National Forensic Science Laboratories (SAPS FSL)7. During 1999, a total of 3971 methaqualone-related cases was submitted to the Laboratory, with the cumulative number of dosage units exceeding three million.
Methaqualone was introduced pharmaceutically in South Africa under the trade name "Mandrax", a formulation containing methaqualone (250 mg) and diphenhydramine hydrochloride (25 mg). Following the identification of its abuse potential, methaqualone and its isomers were effectively removed from the legal market in 19715.
All methaqualone seized in South Africa originates from illicit manufacturing sources in the middle-east, south and central Asia, as well as South and southern Africa5. The product is marketed in South Africa as illicit tablet formulations usually in combination with the antihistaminic drug diphenhydramine, and less frequently with the benzodiazepine tranquilliser diazepam. The formulation of methaqualone with diphenhydramine is thought to be historic in nature with illicit producers simply mimicking the original licit "Mandrax" formulation, or by design due to the fact that diphenhydramine inhibits the metabolism of methaqualone8.
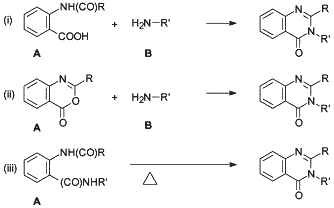
Fig. 2.
Generic reaction schemes for the synthesis
of 2,3-disubstituted-4(3H)-quinazolinones.
Methaqualone abuse gives rise to a barbiturate-type dependence9. The most prevalent abuse pattern observed in South Africa is in conjunction with Cannabis6. This involves mixing methaqualone with Cannabis and then smoking it as a so-called "witpyp", i.e. white pipe.
The synthesis of methaqualone usually involves, but is not limited to, uncomplicated one and two step reactions that are easily adapted for illicit synthesis10. Soliman and Soliman11 stated in 1979 that the majority of 2,3-disubstituted 4(3H)-quinazolinones reported in literature have been synthesized via the following generic routes as depicted in Fig. 2:
- Route (i): Reacting N-acylanthranilic acid (A) with a primary amine (B) and a catalyst in a suitable solvent.
- Route (ii): Reacting 3,1,4-benzoxazones (acylanthranils) (A) with amines (B).
- Route (iii): Thermal cyclization of o-acylamino (N-substituted) benzamides (A).
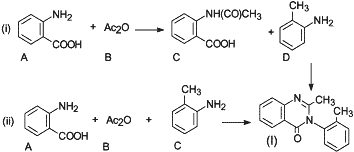
Fig. 3.
Generic reaction schemes for the synthesis of methaqualone.
In 1985, Angelos and Meyers2 reported that the following two basic synthetic routes for the illicit manufacture of methaqualone have been encountered as depicted in Fig. 3:
- Route (i): A two-step reaction involving the preparation of N-acetylanthranilic acid (C) from anthranilic acid (A) and acetic acid anhydride (B), followed by condensation with o-toluidine (D) in the presence of phosphorous trichloride.
- Route (ii): A one-step reaction carried out by refluxing anthranilic acid (A), acetic acid (or acetic anhydride) (B), and o-toluidine (C). Polyphosphoric acid may be added to remove water.
A survey of some important synthesis is given by Ramana and Kantharaj12, and can also be found in limited other sources13,14.
The aim of this paper is to provide a complete and detailed literature survey on the reported synthesis of a methaqualone and some positional and structural isomers thereof.
2. Scope of this survey
The target compounds considered for inclusion in this survey where determined based on the following:
- All compounds must be synthesized via routes that would be suitable for methaqualone synthesis, with only systematic substitution of precursors. The rationale being that these compounds must all be compounds that could, intentionally or accidentally, be produced by illicit methaqualone producing laboratories.
- All compounds had to be isomers — positional and/or structural — of methaqualone. This was considered relevant as the analytical technique of choice to determine methaqualone at this laboratory is coupled gas chromatography — mass spectrometry (GC–MS). Including these isomers in the survey would, thus, give an indication of the likely hood of encountering them in illicit street seizures marketed as methaqualone. This in turn would assist in evaluating the selectivity of existing GC–MS methods in use at the SAPS FSL, as these isomers may then be included as possible interfering compounds during validation studies.
Table 1.
Target 4(3H)-quinazolinones (4(3H)-Q) identified in this survey.
No. |
Target | MM |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
I | 2-Methyl-3-o-tolyl-4(3H)-Q | 250 | Me | Me | H | H | H | H |
II | 2-Methyl-3-m-tolyl-4(3H)-Q | 250 | Me | H | Me | H | H | H |
III | 2-Methyl-3-p-tolyl-4(3H)-Q | 250 | Me | H | H | Me | H | H |
IV | 3-(2,3-Dimethylphenyl)-4(3H)-Q | 250 | H | Me | Me | H | H | H |
V | 3-(2,4-Dimethylphenyl)-4(3H)-Q | 250 | H | Me | H | Me | H | H |
VI | 2-Ethyl-3-phenyl-4(3H)-Q | 250 | Et | H | H | H | H | H |
VII | 3-o-Ethylphenyl-4(3H)-Q | 250 | H | Et | H | H | H | H |
Table 1 list the specific target compounds identified during this survey.
3. Survey
The survey encompasses 32 published papers and registered patents, detailing 39 reported synthesis. In 1946 Grimmel et al.15 reported the synthesis of inter alia compound III, starting from N-acetylanthranilic acid and p-toluidine in the presence of PCl3. This general procedure of condensing a N-acylanthranilic acid with a substituted or unsubstituted aromatic amine, usually in the presence of PCl3, POCl3, or polyphosphoric acid is reported a further 11 times in literature1,10-12,16-22.
Similar to the above route is synthesis of I starting with the hydrochloride salt of o-toluidine which was reported in Dutch Patent 295,501 in 196523, or alternatively by starting with the sodium salt of the N-acylanthranilic acid which was reported by Rawat24 in 1988.
Synthesis starting from anthranilic acid which is acylated and reacted with an aromatic amine was reported in196016, with a further four reports since18,25-27. These proceed via either a one-, or a two-step route, with the intermediates being either N-acylanthranilic acid or acylanthranil depending on the work-up.
Acetanthranil as a precursor for synthesis was reported on in 1963 by Boltze et al.17, with two more reports since28,29. These routes all involved condensation with a substituted primary aromatic amine to yield the target 4(3H)-quinazolinone.
Manhas et al.30 reported the synthesis of I·HCl starting from isatoic anhydride, o-toluidine, and an acetylating agent, detailing a one- and a two-step route. It was subsequently reported twice31,32.
The synthesis of III from p-methylacetophenone oxime and methylanthranilate was reported by Stephen et al.33 in 1956. This reaction proceeded via the di-o-tolylacetamidine intermediate and SOCl2 was used as a reagent.
In 1961, Grammaticakis34 reported on the synthesis of I, II, and III starting from the corresponding N-tolyl-o-nitrobenzamide and an acetylating agent. The synthesis proceeded via the N-substituted-o-aminobenzamide and the N-substituted-o-acylaminobenzamide. Miyata et al.35 reported the synthesis of I starting from N-o-tolyl-o-aminobenzamide and an acetylating agent in 1997.
In Austrian Patent 235,839 (1964), Ecsery et al.36 reported on the preparation of I starting with N-acetylanthranilic acid and various N-o-tolyl compounds, including isocyanate, isothiocyanate, urea, thiourea, thiourethane, and dithiourethane.
The synthesis of I from methylanthranilate, (MgBr)2-N-o-toluidine, and acetic anhydride via N-o-tolylanthranilamide were reported in 196537. In 1967 Hurmer and Vernin38 reported the synthesis of VII·HCl from o-ethylphenyl-
anthranilamide and o-ethylformate, as well as from anthranilic acid and N-formyl-o-ethylaniline. Kozhevnikov et al.39 subsequently reported the synthesis of VI from N-propionyl- o-methylanthranilate and N,N-dimagnesiumhalido- aniline in 1969.
The preparation of I-d4 from phtalimide-3,4,5,6-d4, acetic anhydride and o-toluidine was described by Fentiman and Foltz40 in 1976. The synthesis proceeded via anthranilic acid and N-acetylanthranilic acid intermediates.
In 1980, Nielsen and Pederson41 reported on the synthesis of I from N-acetylanthranilate and o-toluidine hydrochloride in the presence of N,N-dimethyl-
cyclohexylamine. Hilmy et al.42 reported on the synthesis of I, II, and III from o-toluidine hydrochloride and 2-acetylamino-
benzonitrile in the presence of N,N-dimethyl-
cyclohexylamine hydrochloride. A summary of this survey is given in Table 2.
4. Summary
The most reported synthetic routes for 4(3H)-quinazolinones involve the condensation of a primary aromatic amine, or salts thereof, with acylanthranilic acid, or acylanthranil. These compounds are either used as precursors, or prepared as intermediates, or in situ from anthranilic acid.
The second most reported route involves the reaction of isatoic anhydride with a primary aromatic amine and an acylating agent in either a one, or a two-step reaction. A third type of synthesis involves the cyclization of o-acylamino (N-substituted) benzamides. Some other more exotic synthetic approaches have been reported in literature, and many more should be possible. Due to the intricate and/or tedious nature of such routes, the author is of the opinion that it is unlikely that these will be encountered at illicit laboratories.
The data provided in Table 2 can effectively be used as a reference source for forensic scientists investigating illicit methaqualone manufacturing sites, and exhibit material originating from such sites. It furthermore provides a detailed list of precursors and chemicals that needs to be controlled and/or monitored in order to assist in the curbing of illicit methaqualone production.
Table 2.
Summary of reported synthesis of some 4(3H)-quinazolinones
# |
Ref |
Year |
Ta |
Precursors | Reagents Solvents |
Yield |
1 |
[15] |
1946 |
III |
N-acetylanthranilic acid | MePh | 68% |
| p-Toluidine | PCl3 | |||||
| Na2CO3 | ||||||
2 |
[1] |
1951 |
I II IV |
N-acetylanthranilic acid | MePh | I 48% II 60% IV 80% |
| o-Toluidine | PCl3 | |||||
| m-Toluidine | Na2CO3 | |||||
| N-propionylanthranilic acid | EtOH | |||||
| Aniline | ||||||
3 |
[33] |
1956 |
III |
p-Methylacetophenone oxime |
SOCl2 | 69% |
| CHCl3 | ||||||
| Methylanthranilate | Alkalizing agent |
|||||
4 |
[16] |
1960 |
I |
N-acetylanthranilic acid | MePh | 80% |
| POCl3 | ||||||
| NaOH | ||||||
| o-Toluidine | HCl | |||||
| EtOH | ||||||
5 |
[16] |
1960 |
I |
Anthranilic acid | HCl | 70% |
| Acetic anhydride | NaOH | |||||
| o-Toluidine | Carbon | |||||
| EtOH | ||||||
6 |
[34] |
1961 |
I II III |
N-o-tolyl-o-nitrobenzamide | SOCl2, or | - |
| N-m-tolyl-o-nitrobenzamide | H2SO4, or | |||||
| N-p-tolyl-o-nitrobenzamide | (CH3COO)2O | |||||
| Acetylating agent | ||||||
7 |
[17] |
1963 |
I |
Acetanthranil | Ph-H/Me/Cl | 74% |
| o-Toluidine | K2CO3 | |||||
| EtOH/i-PrOH | ||||||
8 |
[17] |
1963 |
I |
N-acetylanthranilic acid | MePh | 74% |
| o-Toluidine | PCl3 | |||||
| EtOH/i-PrOH | ||||||
9 |
[36] |
1964 |
I |
N-acetylanthranilic acid | Xylene or PhNO2 |
- |
| N-o-tolylisocyanate, or | ||||||
| N-o-tolylisothiocyanate, or | ||||||
| N-o-tolylurea, or | ||||||
| N-o-tolylthiourea, or | ||||||
| N-o-tolylthiourethane, or | ||||||
| N-o-tolyldithiourethane | ||||||
10 |
[28] |
1965 |
I |
Acetylanthranil | None | 45% |
| o-Toluidine | ||||||
11 |
[18] |
1965 |
I |
N-acetylanthranilic acid | H3PO4 | - |
| Activated C | ||||||
| o-Toluidine | Na2CO3 | |||||
| MeOH | ||||||
12 |
[18] |
1965 |
I |
Anthranilic acid | H3PO4 | 62% |
| Acetic acid | Activated C | |||||
| o-Toluidine | Na2CO3 | |||||
| MeOH | ||||||
13 |
[25] |
1965 |
I VI |
Anthranilic acid | PhCl | I 90% VI 91.5% |
| Acetic anhydride | POCl3 | |||||
| o-Toluidine | NaOH | |||||
| Propionic anhydride | Na2CO3 | |||||
| Aniline | HCl | |||||
| Activated C | ||||||
14 |
[23] |
1965 |
I I (HCl) |
N-acetylanthranilic acid | HCl | I·HCl 72% |
| NaOH | ||||||
| o-Toluidine·HCl | EtOH | |||||
| Et2O | ||||||
15 |
[37] |
1965 |
I |
Methylanthranilate | NaOAc | 95.3% |
| N,N-dimagnesium- bromido-o-toluidine |
NaOH (calcinated) |
|||||
| Acetic anhydride | EtOH | |||||
16 |
[26] |
1966 |
I |
Anthranilic acid | H3PO4 | - |
| P2O5 | ||||||
| Acetic anhydride | Na2CO3 | |||||
| HCl | ||||||
| O-Toluidine | Activated C | |||||
| NH4OH | ||||||
17 |
[38] |
1967 |
VI I (HCl) |
N-formylanthranilic acid | MePh | - |
| POCl3 | ||||||
| o-Ethylaniline | EtOH | |||||
| HCl | ||||||
18 |
[38] |
1967 |
VII (HCl) |
Anthranilic acid | Na2CO3 | - |
| N-formyl-o-ethylaniline | HCl | |||||
19 |
[38] |
1967 |
VII (HCl) |
o-Ethylphenyl- anthranilamide |
HCl | - |
| O-Ethylformate | ||||||
20 |
[39] |
1969 |
VI |
N-propionyl- o-methylanthranilate |
None | 85% |
| N,N-dimagnesium- halidoaniline |
||||||
21 |
[27] |
1969 |
I |
Anthranilic acid | MePh | 87% |
| Acetic anhydride | PCl3 | |||||
| o-Toluidine | NaOH | |||||
| EtOH | ||||||
22 |
[29] |
1976 |
III |
Acetanthranil | Benzene or Et2O |
- |
| o-Toluidine | ||||||
| p-Toluidine | Basifying agent |
|||||
23 |
[40] |
1976 |
I-d |
Phtalimide-3,4,5,6-d4 | NaOH/Br2/HCl | - |
| Acetic acid | ||||||
| Acetic anhydride | MePh/POCl3 | |||||
| Na2CO3/MeOH | ||||||
| o-Toluidine | Activated C | |||||
| Hexane | ||||||
24 |
[30] |
1977 |
I (HCl) |
Isatoic anhydride | Et2O | 85% |
| o-Toluidine | CH2Cl2 or Hexane |
|||||
| EtOH | ||||||
| Acetylacetone | HCl | |||||
25 |
[30] |
1977 |
I (HCl) |
Isatoic anhydride | MePh | 80% |
| o-Toluidine | HCl | |||||
| Acetylacetone | ||||||
26 |
[19] |
1978 |
I |
N-acetylanthranilic acid | BrPh | 48.4% |
| Benzene | ||||||
| HCl | ||||||
| Et2O | ||||||
| O-Toluidine | NaOH | |||||
| Benzene or Pet. Ether |
||||||
27 |
[20] |
1979 |
I |
N-acetylanthranilic acid | POCl3 | - |
| O-Toluidine | MePh | |||||
| Basifying agent |
||||||
28 |
[11] |
1979 |
I |
N-acetylanthranilic acid | - |
60% |
| O-Toluidine | ||||||
29 |
[31] |
1980 |
I |
Isatoic anhydride | Basifying & Acidifying agents |
- |
| Acetylating agent | ||||||
| O-Toluidine | ||||||
30
|
[31]
|
1980
|
I
|
Isatoic anhydride | POCl3 | - |
| O-Toluidine | ||||||
| Acetic anhydride | ||||||
31
|
[41]
|
1980
|
I
|
N-acetylanthranilate | P2O5 | 84% |
| N,N-dimethyl- cyclohexyl- amine |
||||||
| o-Toluidine·HCl | NaOH | |||||
| CH2Cl2/EtOH | ||||||
32
|
[10]
|
1981
|
I
II
III
|
N-acetylanthranilic acid | MePh | - |
| o-Toluidine | PCl3 | |||||
| m-Toluidine | MeOH | |||||
| p-Toluidine | CHCl3 | |||||
| HCl/NaOH | ||||||
33
|
[21]
|
1984
|
I
(HCl) |
N-acetylanthranilic acid | MePh | I·HCl 76% |
| O-Toluidine | CHCl3/POCl3 | |||||
| MeOH/Acetone | ||||||
34
|
[42]
|
1987
|
I
II
III
|
o-Toluidine·HCl | P2O5 | I 53% II 40% III 33% |
| 2-Acetylaminobenzonitrile | N,N-Dimethyl- cyclohexyl- amine·HCl |
|||||
| NaOH | ||||||
| CH2Cl2/MeOH | ||||||
35 |
[24] |
1988 |
I |
Sodium N-acetylanthranilate |
MePh | - |
| o-Toluidine | PCl3 | |||||
36 |
[22] |
1990 |
I
II
|
N-acetylanthranilic acid | MePh/PCl3 | I 22.8% |
| o-Toluidine | NaHCO3 | |||||
| p-Toluidine | CHCl3/MgSO4 | |||||
| i-PrOH | ||||||
37
|
[12]
|
1994
|
I
II
VII
|
N-acetylanthranilic acid | TosCl/Pyridine | I 75% II 80% VII 68% |
| o-Toluidine | NaHCO3 | |||||
| m-Toluidine | CH2Cl2 | |||||
| N-propionylanthranilic acid | Na2SO4 | |||||
| Aniline | n-Heptane | |||||
38
|
[32]
|
1997
|
I
|
Isatoic anhydride | AcCN/Benzene | 54% |
| o-Toluidine | Activated C | |||||
| Acetylating agent | TsOH/NaHCO3 | |||||
| Pet. Ether | ||||||
39
|
[35]
|
1997
|
I
|
2-Amino-(N-o-tolyl)- benzamide |
Halogenated Trialkylsilane |
- |
| Acetylating agent | Base |
a. Target 4(3H)-quinazolinone(s) reported in reference
following roman numerals as designated in Table 1.