Triboluminescence, the emission of light when a crystal is broken, has been known for some time but remains an obscure phenomenon even though many inorganic and organic materials are reported to exhibit this property.1
The effect has been explained by excitation of the molecule by an electric discharge between the surfaces of the fractured crystal and subsequent fluorescence. In the course of the laboratory preparation of 2-methylbenzisoxazinone,2,3 we have found that its hydrolysis product, N-acetylanthranilic acid, is highly triboluminescent and that this serves to make the experiment more intriguing than the normal synthetic preparation.
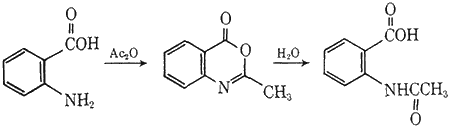
In the experiment, anthranilic acid is converted to 2-methylbenzisoxazinone by the action of acetic anhydride. This compound may be isolated (a good exercise in that the benzisoxazinone is readily hydrolyzed by atmospheric moisture2) or converted directly to N-acetylanthranilic acid by a mild hydrolytic reaction.
Experimental
Preparation of N-acetylanthranilic acid
Place 10 g (0.072 mole) of anthranilic acid in a round-bottom flask equipped with a reflux condenser. Add 30 mL (32g, 0.32 mole) of acetic anhydride, bring the mixture slowly to the reflux temperature and maintain heat for 15 min. Allow the solution to cool and add 10 mL water through the condenser. Bring the mixture to a soft boil once more and allow to cool slowly. Isolate the crystals of N-acetylanthranilic acid (mp 183–185°C) by suction filtration in the hood and wash the product with a small amount of cold methanol.
If the intermediate benzisoxazinone is isolated according to Heimkamp and Johnson2 it may readily be converted to N-acetylanthranilic acid by dissolving in a hot mixture of 35 mL of acetic acid and 10 mL of water (assuming approximately 10g of the benzisoxazinone) and allowing the solution to stand. These procedures usually yield well-formed crystals but the material may be recrystallized from acetic acid/water mixtures.
To demonstrate the property of triboluminescence, the crystals should be well-formed, and it is vital that they be completely free of solvent. The triboluminescence is best demonstrated by placing several crystals of the compound between two watch glasses and gently grinding. The light emitted is readily observed in a darkened room.