Chemistry
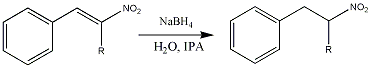
The reduction of the double bond in nitrostyrenes has been a great source of pain for many researchers. It has traditionally been reduced by various metal borohydrides in yields varying from bad to great. The high-yielding methods has relied on huge excess of reducing agent, and in some instances huge amounts of solvents. Thus making these methods somewhat unattractive despite the good yields. Phase transfer catalysis (PTC) can be used to transfer reagents (or substrates) from a phase where it is soluble to a phase where it is insoluble. Using PTC a reaction can take place in a solvent in which it normally couldn't.
After some additional work Barium found out that a PTC was not even required. IPA could be used as a solvent and works as a pseudo-PTC as the same time
General Procedure
Use the following amounts of reagents:
- 1 mole eq. of any arylnitrostyrene
- 1,25 mol eq. potassium or sodium borohydride1
- Approximately 5*wt. of the nitrostyrene in mL's of IPA (i.e. 10 g P2NP would require 50 mL IPA)
- 2/5 the vol of IPA used of dH2O
Mix the IPA and water in a sufficiently large beaker. Add the borohydride in one portion and commence stirring. Begin adding the nitrostyrene in small portions after a minute or two. This will cause a rise in temperature and moderate hydrogen evolution. Add the substrate in small portions to avoid any nasty volcano reactions. The rate of addition should be such that a lively evolution of hydrogen can be observed in the beaker, but slow enough to avoid the reaction shooting out of the flask.
Once all of the substrate has been added, keep stirring the mixture for 30 mins. The colour of the mixture should be alot more pale than that of the nitrostyrene2. Add dilute (32-80%) acetic acid drop wise untill fizzing stops. Add solid non-iodized table salt while stirring heavily, untill no more dissolves. Suction filter the mixture, to remove any remaining salt and borates. Rinse the filter cake with a little fresh IPA. The IPA layer, containing the product will float on top of the water. Isolate the IPA layer, and discard the water.
At this point the reaction is over, and the IPA layer contains a product which is sufficiently pure for a CTH, Zn/Formate, SnCl2 nitro reduction or whatever your preference might be. So simply use this IPA direcly.
Experimental
Reduction of 2,5-dimethoxy-beta-nitrostyrene to 2-nitro-1-(2,5-dimethoxyphenyl)ethane
The following reagents/solvents where used:
- 10,5 g (50 mmol) 2,5-dimethoxy-beta-nitrostyrene
- 2,4 g (63 mmol, 1,25 mol eq.) sodium borohydride
- 50 mL IPA
- 20 mL dH2O
The reduction
10,5 g (50 mmol) 2,5-dimethoxy-beta-nitrostyrene was added during 5 minutes to a solution of 2,4 g (63 mmol, 1,25 mol eq.) sodium borohydride in 50 ml IPA and 20 ml water. The temperature rose from 20 to 50°C while the orange color faded to a slightly yellow. Dilute 80% acetic acid was added untill no more fizzing from the remaining borohydride occured. This is followed by addtion of enough solid NaCl to cause the IPA to form a separate upper layer containing the product. The mixture was suction filterd and rinsed with a little IPA.
Non-mandatory isolation of the nitroalkane
This section is included to give an estimate of the yield in this first reduction. It is not nescassary to perform this step unless the nitro alkane is the target compound.
Two volumes of water was added to the isolated IPA layer which caused 2-nitro-1-(2,5-dimethoxyphenyl)ethane to separate as a clear yellow oil and was isolated by extraction with 2x15 ml toluene. The organic phase dried over MgSO4 and the solvent removed under vacuum leaving the product as a clear yellow oil. Yield 9,7 g (46 mmol, 92%) 2-nitro-1-(2,5-dimethoxyphenyl)ethane.
Conclusion:
There is hardy any reason to optimize this reaction any further. All of the reagents are cheap, relative non-toxic and the yields are very high. The reaction itself is very easy to perform and there is little chance of failure.
Combined with a proper nitro reduction, this method gives anyone who would desire it, a very easy route to some of the aminoalkanes people seem to like. So i believe its pretty safe to send LAH back to the sulfur reeking pit from where it came.