Chemistry
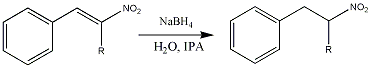
Reducing the double bond with sodium borohydride in IPA and water, will give the nitroalkane in high yields. The IPA layer isolated from the reduction is topped up with 20% more IPA and used directly in the next step.

The IPA containing the nitro compound is simply mixed with the 5% Pd/C, and a premade slurry of KOH and HCOOH is added. The whole mess is stirred at 75-80oC for a few hours, whereafter the reaction is over. The nitrogroup is reduced with potassium formate as the hydrogen donor. Potassium gives much superior yields to ammonium and sodium. The Pd acts as a hydrogenation transfer catalyst. The amount of water present is very critical for both the yield and the swiftness of the reaction. A 2.5-3 molar equalivent of water to formate usually gives the highest yields. The reaction rapidly and is usually over in 1-3 hours. Once hydrogen evolution ceases the reaction can be considered done.
Experimental
The Reduction
This example uses (p-fluoro-phenyl)-2-nitropropane as the substrate. This can of course be substituted with any other similar nitroalkane and applied in the same manner.
The following amounts where used. The amount of the nitropropane can simply be switched with the amount of nitropropene used, as the first reduction is so high yielding.
- 50 mmol nitroalkane
- 250 mmol potassium formate1
- 750 mmol water
- 25-50 ml IPA (depending on how much is needed to get a nice solution)2
- 10%w/w 5%Pd/C (catalyst to substrate)
The IPA layer from the previous reaction was topped of with +20% more to make it a little more dilute. 0.7 g of 5% Pd/C was added to the IPA layer and stirred heavily.
In another beaker 7 mL's of water was made into a slurry with 14 g's of dry KOH. While stirring like a madman, 13.5 g 85% HCOOH was dripped in (caution: very exothermic). Do the math yourself on how much water to add. You get water from: the neutralization of the two acids, the water in the formic acid, the KOH and perhaps the Pd/C if you use prewetted.
The potassiumformate mixture was poured into the nitro/IPA flask in one portion. The whole lot is heated to 75oC while stirring. Evolution of hydrogen was noted around 50oC. A few mL's of 80% acetic acid was added now and then, when the mixture got too "lumpy" (4 mLs was used in total). After two hours the hydrogen evolution had subsided and the reaction was deemed over.
Workup
The black slurry was filtered twice through filter paper (first time alot of black sludge came through, but simply use the same filter as before as a "plug" for the second filtering). The filtercake was rinsed with a little IPA to get all the goodies with it. The final filtrate was almost colourless. After saving the filtrate, the filtercake was washed with water, some dilute hydrochloric acid, and finally some pure water to remove acid traced. This can then be scraped up and saved in the "used 5% Pd/C pile".
The almost-clear filtrate was saturated with table salt and filtered (the Pd/C filtercake can actually be re-used here and then cleared up with water). The lower aqeous layer was yet again discarded and the IPA stripped at atmospheric pressure.
The yellowish oil residue weigthed 6.5 grams. This was dissolved in dilute hydrochloric acid and washed twice with 15 mL's DCM, giving a nearly colourless solution (perhaps a tint of green/yellow?). The aqeous layer was basified and extracted with 3x20 mL DCM. The DCM was dried with a tiny bit of MgSO4, filtered and stripped. This left 4.5 grammes of a clear oil (p-F-amphetamine). The yield the total reduction is 72% with very easy steps in between .
The p-F-amphetamine was dissolved in IPA, and precipitated with a precisely half molar amount of H2SO4 dissolved in IPA. This was added in a thin stream and when the whole mess was unstirrable it was filtered and the filtrate titrated with the acid again. Yield of the sulfate salt was quantitative (95%+), which is good enough for me ! GC-MS analysis showed 98%+ purity of the compound, which is as high as that particular CG-MS goes. Thus no distilliation of the amine is nescassary.
Conclusion:
A similar reduction of (2,5-dimethoxy-phenyl)-2-nitropropene gave an overall yield of 86% in the two step reduction. The method followed was exactly the same as described above
With a little practice the reduction from the nitropropene to the amphetamine can be done in about five hours total. The yields are really good, and reaction conditions are mild. It does not seem to bother the reactions, that the same IPA is used throughout the scheme. So please do reduce away :-)
Pretty slick dare i say!