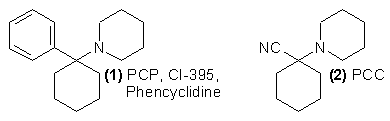
The original chemistry laying the groundwork for the preparation of phencyclidine (PCP, 1) was a study reported in 1926 describing the reaction of 1-piperidinocyclohexanecarbonitrile (PCC, 2) with Grignard reagents1. It was not until some 30 years later that the anesthetic effectiveness of (1) was observed in animals2, and the compound was made available by Parke Davis and Co. under the name of Sernyl (CI-395) for clinical investigation in man.
The first surgical studies described the development of complete analgesia within a few minutes following intravenous administration of approximately 20 mg, but at the higher dosages required for surgical anesthesia (some 4x this amount) there was observed an excited state which required supplementary pentobarbital for control3. Other early observers of PCP use in clinical anesthesia had reported related undesirable side reactions, both during the operative state as well as in recovery. Johnstone et al.4 made comment concerning the patient's volunteering of information suggesting experiences of trance-like ecstatic states and, upon emergence a euphoria accompanied by hallucinations and visual distortions. Similarly, Riffin reported post-operative side reactions including dizziness, slurred speech, and manic behavior5.
It was this constellation of consistent and undesirable properties of the drug that prompted its removal from clinical use in 1965, and which most certainly fostered and supported its subsequent street acceptance. It was widely distributed and used in San Francisco as "Peace Pill" and "Hog" in 1967 ("Peace Pill" was derived by the pronouncing of the consonants PCP, themselves an abbreviation of the complete chemical name for (1), phenyl-cyclohexyl-piperidine). Since that time the drug has been consistently available, both under its own name as well as with misrepresentation as any of a number of other drugs which might enjoy momentary popularity.
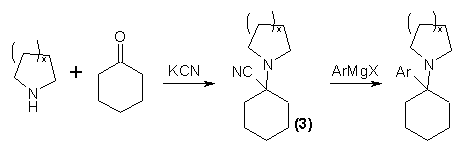
The published preparations of PCP and its analogs may be considered in three groups: those which employ the nitrile (2), those which depend upon the formation of a Schiffs' base as an intermediate, and those which invoke an enamine compound. The most important and most frequently used route to PCP and to its analogs which maintain a cyclic amine function, exploit the facile displacement of the nitrile group from the intermediate (3) with an aryl Grignard reagent6. This method is completely general for cyclic secondary amines (as piperidine and C-alkylpiperidines, pyrrolidine and alkylpyrrolidines, morpholine, and methylpiperazine) and aromatic Grignard reactants (as phenyl, halophenyl, tolyl, anisyl, trifluorotolyl, and thienyl)6,7.
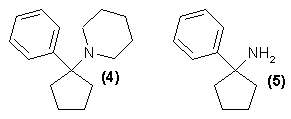
The intermediate cyclohexanecarbonitrile can be prepared from the bisulfite addition product of cyclohexanone by treatment with KCN and piperidine6, or by the addition of cyclohexanone and KCN to an aqueous solution of piperidine hydrochloride7. Both procedures give excellent yields. The Grignard reaction is conventional, being worked up either with HBr or NH4Cl. The use of phenyl lithium in place of phenyl magnesium bromide results in addition to the nitrile rather than in its displacement. Efforts to prepare the cyclopentyl homolog (4) of PCP employing this procedure also failed with phenyl lithium brand this compound could only be made by the construction of the piperidine ring from the primary amine (5) with pentamethylene bromide7.
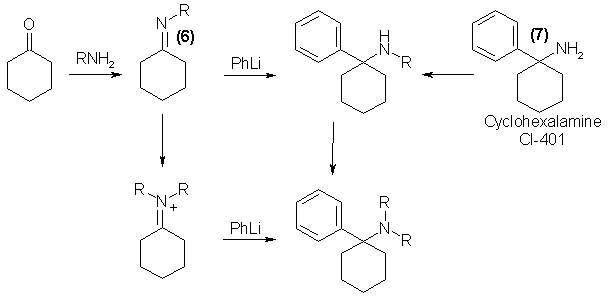
Analogs of PCP with a mono- substituent on the nitrogen are best prepared through the Schiff base intermediate (6) formed from cyclohexanone and the appropriate primary amine6. The introduction of the phenyl group can be achieved with phenyl lithium. The N,N-dialkyl derivatives are easily prepared from their monoalkyl counterparts by methylation or ethylation via N-formylation or N-acetylation and reduction with lithium aluminum hydride (6). In addition, the free amino compound (7) can be employed for the synthesis of either mono- or dialkyl products; amide formation followed by reduction yields monoalkyl isomers, and reductive methylation (with formaldehyde and formic acid) will provide dimethylation7. If the Schiffs' bases are reacted with an alkyl halide, a quaternary salt results, and this, upon reaction with phenyl lithium, will provide a mixed N,N-dialkyl analog of PCP.
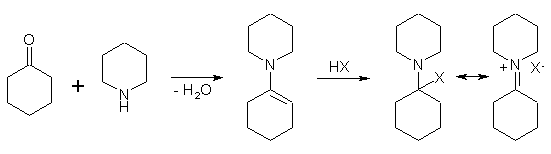
Yet another scheme is known for the synthesis of this class of compounds. An enamine is prepared by the dehydration of equimolar quantities of piperidine and cyclohexanone. The addition of anhydrous p-toluene sulfonic acid or of hydrogen bromide to this system produces an adduct from which PCP can be obtained by displacement with a Grignard reagent9.
The majority of the illicitly synthesized PCP is prepared according to the general directions of Kalir et al.7, modified in details as required by the availability of chemicals and equipment. A typical batch operation will be on a 3- to 5-mole scale, and is usually limited by the amount of piperidine to be used (usually a maximum of 500 gm). Of all the reagents needed in this preparation, this last is the most difficult to obtain and can command as much as $1000/kg for a clean bottle (i.e., one which is not watched or traceable). The procedure given in Ref. 7 for the preparation of PCC (2) is followed religiously, except that if the product does not crystallize spontaneously after overnight standing the reaction mixture is extracted either with benzene or with white gasoline, which is then dried with an appropriate anhydrous salt before evaporation. The most common choices are sodium sulfate, calcium chloride, and potassium carbonate.
The Grignard reagent employed in the second half of the reaction (phenyl magnesium bromide) is inevitably prepared within the laboratory, as it is felt that commercially prepared material is carefully watched. Phenyl bromide is used, ether is the invariable solvent, and elemental iodine is popular for initiating the reaction. The isolated nitrile is usually dissolved in white gasoline for addition to the formed Grignard solution, and the latter is always used in excess of stoichiometric requirements. A 1.25 to 1 ratio is minimum, but if the Grignard can be used in an excess of 2 to 1, the yield of the final product can be as high as 65%, based upon the amount of piperidine employed. The workup is as described in Ref. 7, except for the usual use of white gasoline in place of ether for extraction purposes. The product is converted to the hydrochloride salt either by the addition of a calculated amount of concentrated aqueous HCl followed by air evaporation, or by the passage of anhydrous HCl into an ether solution of the base. Recently the free base itself has appeared on the street.
In neither of these two work-ups is unreacted nitrile (2) satisfactorily removed, and its unexpected toxicity can have serious consequences both to the synthetic chemist and to the eventual user. These points are discussed below.
Of the many analogs of PCP which have been chemically prepared and described, as of the present time only a few are known to have appeared in illicit street use. The thiophene analog TCP (8) was first identified by the San Francisco Drug Enforcement Administration laboratory in 197210. In the illicit synthesis of TCP the precursor 2-bromothiophene is the limiting reagent (both as to expense and availability). Although the product is somewhat more potent than PCP, it is sold 1:1 on a weight basis. Abuse of this isomer spread quickly and widely, and it was entered into Schedule I of the Controlled Substances Act in July, 197511.

The N-ethyl analog of PCP (9), PCE, cyclohexamine, CI-400 of Parke Davis and Co.) first appeared in the Los Angeles area about 1969. The initial samples were a wet yellow to brown gum, and were sold and accepted as PCP. The average dose was somewhat smaller than PCP, however, suggesting a higher human potency. This is in keeping with reported animal data7. By 1971 this drug was available as a white solid, superficially indistinguishable from PCP hydrochloride; at no time was it identified other than as PCP. A single report in 1970 mentions the discovery of a clandestine laboratory presumably synthesizing this isomer12.
Another early substitute for PCP was the pyrrolidine homolog (10). Although both mouse and primate screening have shown this compound to be quantitatively and qualitatively similar to PCP7, it was found in street use to cause a barbiturate-like sedation, and it was not accepted. A recent report13 mentions this compound as having appeared in New Jersey, dispersed on vegetable matter intended to be smoked. Neither the N-ethyl nor the pyrrolidine analogs of PCP are presently recognized in the Controlled Substances Act schedules.
Considering the usual methods employed in illicit manufacture of PCP or closely related analogs, a general picture can be drawn of the variety of chemicals that might, taken together, suggest such purpose. At least one chemical from each of five chemical groups must be represented, as well as the more usual chemical accessories such as solvents and drying agents.
- An aliphatic amine: examples would be piperidine, EtNH2, pyrrolidine, morpholine, N-methyl-piperazine.
- An aliphatic ketone: cyclohexanone.
- An aromatic halide: examples would be bromobenzene, bromoanisole, bromotoluene, bromothiophene.
- A leaving group intermediate: examples would be Potassium cyanide, HBr, p-toluenesulfonic acid.
- A metal: magnesium or lithium.
As both the intermediate nitrile PCC (2) and the final product (such as PCP) have similar basicity and solution properties, any incomplete conversion will result in a product containing varying amounts of PCC as a contaminant. This chemical may well be implicated in the toxic complications associated with PCP use14. Immense care is taken during illicit manufacture at the stage where this intermediate is collected and dried, more even than in the handling of the final product. Chronic exposure to this compound has led to an aggravated psychotic condition that has resulted in a protracted hypersensitivity to the chemical, as shown by skin-patch assay15. The extent of contamination of PCP with this intermediate is difficult to determine, as most analytical schemes (GLC, TLC) appear to promote its decomposition. A technique has recently been developed allowing estimation of PCC in PCP and TCP16. A major laboratory, which has had extensive experience in the analysis of street drugs, has found that in all samples submitted for analysis which proved to be PCP, approximately 20% contained detectable amounts of PCC17.
There is a defensive value in briefly reviewing the published scientific literature that exists concerning related compounds, for it may well be from these papers that clues can be obtained concerning tomorrow's pharmacologic and analytic problems. In the references already cited, there are literally dozens of analogs described, both chemically and pharmacologically. Implicit in the generalized directions are dozens more, many of which may reasonably be predicted to be of similar pharmacology. A host of analogs with the aromatic ring replaced with other functions such as benzyl, vinyl, allyl, acetyl, enyl, and cyano groups have been studied as anticholinergic agents, and appear to be similar in potency18-20. A related class of compounds that has already been clinically established as being pharmacologically equivalent to PCP, can be illustrated by the structure of ketamine (11). Although somewhat less potent than PCP, it produces at effective doses a depersonalization and intoxication state which, through word of mouth, is being touted as being superior to that produced by PCP. The thiophene analog of ketamine, tiletamine (12), is currently also in clinical study, and a large number of related analogs have been prepared.
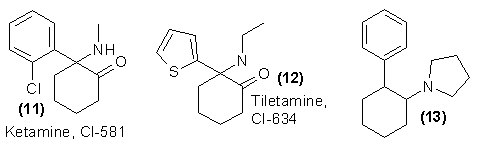
There must also be considered the continuing flood of new compounds in the literature, chemicals which are less closely related in structure, but which are described as producing sensory disturbances, disorientation, and related symptoms. An Arbitrary single example of such a compound is (13)21. Although such dysphoric properties are considered as undesirable side reactions and reason for the discontinuation of study of a given drug, these very properties are the ones that attract the attention of those individuals who are the advocates of this form of drug abuse. The pharmacologic literature is avidly followed, and there are, apparently, an unending line of experimental subjects who, knowingly or unknowingly, can be called upon to determine the properties of an unknown drug.