Introduction
Our present understanding of the mechanism of action of the psychotomimetic drugs has come from studies in several disciplines. Physicochemical parameters of active compounds are easily measured and may reflect structural aspects which influence absorption and distribution in the experimental subject. In metabolic studies, the fate of these compounds has suggested possible intermediate active species, or the formation of detoxification products. Pharmacological research has revealed a complexity of neurotransmitter involvement during the course of their action. In intact animal studies the behavioral changes induced by these drugs have allowed prediction of potential activity levels in clinically untested compounds. A number of experimental animal model systems have shown a good correlation between performance modification and demonstrated human psychotomimetic potency.
The only effective challenge to the accuracy of predictions from such in vitro and in vivo studies comes from the eventual evaluation of the studied compounds in clinical trials in normal human subjects.
Three compounds are described in this presentation. Although all are known chemicals, they have not been previously reported to be psychotomimetic in man. They are structurally unrelated to one another, but each is a close analog of a recognized family of hallucinogens.
4-Methylthio-2,5-dimethoxyphenylisopropylamine (para-DOT, 3)
The para-dimethoxy substitution pattern is a structural feature frequently associated with high psychotomimetic potency. Such compounds are easily converted to quinones, and can potentially provide to the body a new chemical species not usually encountered in metabolic chemistry. Such an intermediate can undergo a number of chemical reactions with preexisting biochemicals such as amines or mercaptans. With substituted derivatives of amphetamine, this methoxy orientation requires a 2,5-disubstitution pattern. In the case of 2,5-dimethoxy-4-methylphenylisopropylamine (DOM, 1c) the metabolically generated hydroquinone has been shown to undergo facile oxidative cyclization to the indoline 21. This metabolic scheme may apply broadly to 2,5-dimethoxyphenylisopropylamine derivatives which vary in the identity of the substituent in the aromatic 4-position. A number of these compounds are established psychotomimetic agents, and can be compared with one another in potency and chronology of action as shown in Table 1.

 | Code | Human Effective Range (mg) | Duration | |
1a |
-H | 2,5-DMA | 30-50 | Short |
1b |
-OCH3 | TMA-2 | 10-30 | Inter- mediate |
1c |
-CH3 | DOM/STP | 1-5 | Long |
1d |
-C2H5 | DOET | 1-3 | Long |
1e |
-Br | DOB | 0.2-1.0 | Long |
2,4,5-Trimethoxyphenylisopropylamine (TMA-2, 1b) can, by replacement of the 4-oxygen atom with the isovalent sulfur atom, give rise to the para-desoxythio (DOT) isomer para-DOT2. The octanol-water partition coefficients of known psychotomimetics have been correlated with their human potencies3,4. The value obtained for para-DOT (3) suggested that it might have 30x the potency of mescaline2. A comparison of TMA-2 (1b), para-DOT (3) and DOM (1c) by the spectrophotofluorometric assay as described by Autun et al.5 gave the relative intensities 0.5, 0.6 and 1.0 resp.6

These physical measurements suggest that para-DOT (3) may lie between 1b and 1c in potency. The psychopharmacologically effective dose of para-DOT in man is in fact intermediate to that of TMA-2 and DOM. Employing the clinical assay protocol described earlier7 the threshold and active levels of para-DOT in normal experimental subjects appear to lie between 5 and 15 mg., administered orally. The chronology of the induced intoxication is similar to that of TMA-2. Initial effects are noted at just over one hour following administration, with a maximum effect reached at about the end of the second hour. Effects maintain a plateau here for about 1½-2 hours, and are completely dissipated by the end of the sixth hour. Qualitatively there were few visual effects reported with para-DOT, but in other respects many of the conceptual and interpretively disruptive aspects of LSD intoxication were induced. With closed eyes, there was an easy visualization of hypnogogic images which, although not under voluntary origin as to subject matter, could be terminated at will.
3,4-Methylenedioxymethamphetamine (MDMA, 4)

A second compound to be described in this presentation is the N-methyl homolog of a well-studied psychotomimetic, 3,4-methylenedioxyphenyl- isopropylamine (MDA, 5).
As with MDA, MDMA (chemically properly N-methyl-3,4-methylenedioxyphenylisopropylamine or 1-(3,4-methylenedioxyphenyl)-2-(methyl- amino)-propane) has the aromatic substitution pattern of the essential oil safrole (6) from which it was first synthesized in 19608. The only toxicological and behavioral report involving this compound was an Army Chemical Center study performed in the 1950ís, declassified in 1967, and published in 19739. There are no reports concerning its psychopharmacological action in man, although the compound has had occasional and erratic appearance in the illicit Street drug market10. For this reason, if for no other, it falls under the purview of the National Institute of Drug Abuse, and a brief description of the pharmacological properties of this compound in man would seem appropriate.
MDMA has a higher threshold level than does MDA, but otherwise it is very similar in potency. Within the effective dosage range, 75-150 mg orally, the effects are first noted very quickly, usually within a half-hour following administration. With most subjects the plateau of effects is reported to occur in another half-hour to hour. The intoxication symptoms are largely dissipated in an additional 2 hours except for a mild residual sympathomimetic stimulation which can persist for several additional hours. There are few physical indicators of intoxication, and psychological sequelae are virtually non-existent. Qualitatively, the drug appears to evoke an easily controlled altered state of consciousness with emotional and sensual overtones. It can be compared in its effects to marijuana, to psilocybin devoid of the hallucinatory component, or to low levels of MDA.
α,O-Dimethylserotonin (α-Methyl-5-methoxytryptamine, 11)

The third psychotomimetic compound to be discussed in this presentation results from minor modifications of the chemical structure of the neurotransmitter serotonin (7). Compounds such as serotonin which are active within the central nervous system (CNS) are not centrally active when administered peripherally. The reasons for this inability to enter the CNS are evident in the nature of the functional groups present. The free hydroxyl group at the 5-position is a highly polar site which appears to effectively prohibit CNS penetration. The rare exceptions to the general rule that phenols and phenol-like compounds are centrally inactive, are usually examples wherein there is possible intramolecular hydrogen bonding involving this acidic function (an adjacent furan oxygen in the case of morphine, ready access to the neutralizing amine function in the case of psilocin). A commonly encountered stratagem found to be effective in circumventing this obstacle is to form a derivative such as an ester (as an acetyl group) or as an ether (as a methoxyl group).
A primary amine function, on the other hand, is generally labile to enzymatic removal, thus leading to inactivation of the drug during its absorption period and prior to its availability to the CNS. A procedural circumvention of this problem is frequently achieved by the parenteral administration of a drug, but a number of structural modifications are also known to be effective. The addition of a carboxylate group alpha to the amine function generates an α-amino acid which can gain entry to the CNS by active transport systems specific for this structure. Decarboxylation in situ then reveals the amine. Conversely, the amine can be effectively shielded from enzymatic deamination by the substitution on it of sterically bulky groups (such as N,N-diisopropyl or an N-tert-butyl structure) or by substitution adjacent to it of a methyl group (as seen in the relationship between phenylethylamine and amphetamine).
α,O-Dimethylserotonin (11) is a compound that meets these structural modifications exactly. It was first prepared in 195811, and has been shown to be both pharmacologically similar to serotonin in in vitro studies12 and centrally active in mice13 It is also closely related to the three known psychotomimetic agents N,N-dimethyltryptamine (DMT, 8), α,O-methyltryptamine (9), and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT, 10). A comparison of the structures and potencies of these compounds in man is shown in Table 2, and it is gratifying to see that the logical extrapolations from these three drugs agree closely with the properties reported here for 11. The replacement of the N,N-dimethyl substitution pattern of DMT with an O-methyl (as in 9, α-methyltryptamine) results in a slight increase in potency, but more importantly, allows the compound to be effective via the oral route. The retention of the N,N-dimethyl system but the addition of a 5-methoxy group (as in 5-MeO-DMT, 10) maintains the requirement of parenteral administration, but results in a several-fold increase in effectiveness.
Table 2
Potencies and Effective Routes of Administration of Several
Psychotomimetic Tryptamine Derivatives in Man
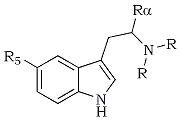 | R | Rα | R5 | Name | Dosage | Route |
Me | H | H | DMT (8) | 30-75 mg | (parenteral) | |
H | Me | H | α-Methyltryptamine | 20-50 mg | (oral) | |
Me | H | MeO | 5-MeO-DMT | 5-10 mg | (parenteral) | |
H | Me | MeO | α,O-Dimethylserotonin | 1.5-3 mg | (oral) |
α,O-Dimethylserotonin (11), as shown in Table 2, is indeed orally active and has an effective dose range of 1.5-3 mg. Unlike the parenterally active drugs, but like the unmethoxylated analog 9, 11 has surprisingly long duration of action. Its use is generally characterized by extensive physical discomfort. Within the first hour following administration there is nausea, frequently accompanied by active vomiting and related stomach cramps. In the following period, during the time of central intoxication (2-6 hours), there is reflexive mydriasis, some instances of diarrhea, and occasional complaints of difficulty in urination. After the majority of the central sensory effects disappear there can be a residual headache, and both irritability and insomnia.
The subjective effects of α,O-Dimethylserotonin are difficult to characterize. As has been observed in several of the reported studies of DMT, the intoxication appears to be largely unstructured, and will vary in nature with the individual subject, almost as if he were bringing to the experiment his own preference in the style of psychotomimetic action. The two published reports on the clinical effects of α-methyltryptamine14 suggest a variety of evoked responses, mostly unaccompanied by overt impairment of mental function. It seems probable that additional study with α,O-dimethylserotonin may show a similar complexity of intoxication. Some Comments Concerning the Need for Acute Studies of Psychotomimetics in Human Subjects
There has been a move in the last few years away from human titration of potential psychotomimetic drugs in favor of biochemical and behavioral studies in animals. The need for animal toxicology and metabolic information is no less than it has ever been, but it must be remembered that the ultimate purpose of research in this area is to alleviate human illness and to provide for human needs. One principle rationale for such studies is to gain insight into the mechanisms of mental illness or, through the development of an understanding of the relationship between structure and activity, to design and assay drugs which might reverse endogenous depression or other undesirable mood states. However, such conditions - schizophrenia, neurosis, autism - are uniquely human complaints. No animal model has yet satisfactorily imitated them. To an extent their symptoms can be pharmacologically induced in normal human subjects, but potentially therapeutic drugs can only be verified in man. A less frequently mentioned goal is the search for compounds which might enhance creativity, or provide entertainment15. Again these requirements are uniquely human, and the determination of risk and benefit from such pharmaceuticals must be made in man as the test animal.
There appears to be an intangible but surprisingly effective prohibition of human research in this area. There is concern with regard to the narcotics laws, and problems associated with the Drug Enforcement Administration in the approval of research protocols involving scheduled drugs. Although the DEA claims to provide no impediment to such research, there are extensive hurdles which must be overcome nonetheless. Most of the potentially valuable drugs to be explored in these areas are not drugs of established abuse potential, are not recognized in the drug schedules, and are consequently of no concern to the DEA. A more pervasive inhibition comes from the HEW guidelines which were originally intended to apply to human experimentation supported by grants and contracts to universities. These have now become routinely consulted by human health committees approving clinical research, regardless of the source of funds. These guidelines state that there must be potential therapeutic value, and that there be a high benefit-to-risk ratio, in any human study. Since it is widely held that work with the psychotomimetic drugs cannot have value and therefore only presents risks, this approval is rarely obtained. The recourse to animal models for exploratory research embodies an element of hypocrisy in that all such studies inevitably depend upon human assessment of pharmacological qualities and potency for achieving a correlation. Further, their value in prediction can only be confirmed by human assay, yet there is often an aversion to such experimentation.
There has been, and will continue to be, much human experimentation with potential hallucinogenic or psychotomimetic drugs. Much of it is self-experimentation, by cautious professors of pharmacology, curious industrial toxicologists, and adventurous graduate students. Most of the results of these inquiries are unpublished and unavailable to the scientific community. At the same time, many of the rumored drug-effects are anecdotal, anonymous, and completely unreliable. Far too often, new drugs with a dramatic word-of-mouth reputation appear in social use, often in some misrepresented form, and providing an extremely high potential for tragedy.
What can be done to remedy this situation? It might be valuable to consider forming a set a human research standards geared to the study of drugs in acute trials, rather than to chronic studies intended for therapy. Such studies might be directed to the disruption of sobriety as the desired property rather than an undesirable side-effect. The subtle stigma associated with the use of experienced subjects should be erased. Ethical considerations limit the exposure of naive subjects to new sensory experiences. Also, their unfamiliarity with the nature of possible subjective phenomena to be encountered can make communication difficult. The objections raised to self-experimentation might be reconsidered, as this provides the epitome of informed consent and the most desirable consistency in the subjective comparison of two different materials.
We cannot afford to wait until an enterprising illicit drug manufacturer successfully markets some new drug, one which might receive enthusiastic public acceptance. We need to be prepared to recognize the symptoms which might be seen in acute intoxication involving such a new drug. We must be familiar with the psychological complications which can accompany exposure to overdosage. Studies of this drug in animal models will provide a wealth of factual information in areas of toxicity and behavior disruption, but this is of utterly no use to us when the drug suddenly appears and is used in society. The research which might provide practical help in such an emergency is not being pursued. We must accept the responsibility to remedy this situation.