Synthesis of 2-Bromo-4,5-dimethoxybenzaldehyde[ Back to the Chemistry Archive ] IntroductionIt has already been covered on this page how to manufacture 3,4,5-trisubstituted benzaldehydes from the cheap and non-suspicious precursor Vanillin (3-methoxy-4-hydroxybenzaldehyde)[4]. Using the information in this document, 2,4,5-trisubstituted benzaldehydes can also be manufactured from vanillin. The outline of the synthesis is to first alkylate the phenol group of vanillin (with MeI or Me2SO4) to form veratraldehyde (3,4-dimethoxybenzaldehyde) or to use another alkyl halide if another substituent than a methoxy group is desired. Then the 3,4-dialkoxybenzaldehyde is brominated as described below to form the 2-bromo derivative (the first synthesis is adapted for a large scale, but can be scaled down as desired). After the 2-Bromo-4,5-dimethoxybenzaldehyde has been prepared, it is reacted with sodium methoxide in methanol, with EtOAc/CuBr as catalyst [4] to form 2,4,5-trimethoxybenzaldehyde, a precursor for TMA-2[5]. Other alkoxides can be substituted for the sodium methoxide to form other alkoxy derivatives. It should also be possible to use thiolates to form the corresponding thio derivatives. The plain 2-Bromo-4,5-dimethoxyphenethylamine (and the corresponding amphetamine) should also be active compounds. 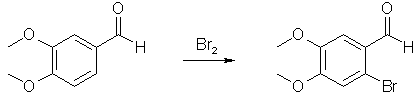
2-Bromo-4,5-dimethoxybenzaldehyde [1] Bromination of 3,4-dimethoxybenzaldehyde in acetic acid has been reported previously [2]. By substituting methanol for acetic acid, we obtained 2-Bromo-4,5-dimethoxybenzaldehyde in 90-92% yield. While it has been reported that reaction of bromine with methanol may be vigorously exothermic, on the scale indicated in our experimental only a mildly exothermic reaction occurred (temperature increase from room temperature to about 40°C) when bromine was added with water-cooling. On a 50 g laboratory scale in experiments using the same concentrations as the 4.0 kg experiment, designed to identify potential hazards, bromine was added at once without cooling, resulting in an exotherm, but the reaction temperature never exceeded 45-50°C. The product contained less than 1% of the 3-bromo-4,5-dimethoxy-benzaldehyde as determined by HPLC and NMR. This procedure has been scaled up to 100 kg quantities with similar yields of 90-92%. Ethanol was less suitable because of decreased solubility and lower yields. A 30-L (nominal capacity: 30 L, total capacity: 45 L, manufactured by Schott) glass reactor is charged with 25 L of methanol, and mill-powdered 3,4-dimethoxybenzaldehyde (4.0 kg, 24.07 mol) is added with stirring, resulting in a 5-8°C temperature drop. The filling tube is rinsed with 2 L of methanol and closed. The mixture is heated to 30°C, if necessary, to achieve a homogeneous solution. Bromine (4.4 kg, 27.53 mol) is added, with cooling (T<40°C), followed by stirring at this temperature for 1 h. The reaction mixture is then heated under reflux to remove 9.5 L of methanol by distillation. At this point, the product may start to precipitate from solution. After cooling to 20°C, 15 L water is added with stirring. The resultant slurry is filtered using a 60-L pressure filter and washed with cold methanol (3x5 L). The colourless to slightly yellowish product is dried in vacuo at 50°C and 40 mbar for 18-24 h. Product yield is 5.4 kg (91.4%), mp 143-146°C (lit. 149-150°C). TLC: Rf = 0.55 (hexane/ethyl acetate, 6:4). 2-Bromo-4,5-dimethoxybenzaldehyde [3] Typical Procedure. A solution of bromine (3.2 mL, 62.5 mmol) in dry chloroform (8 mL) was added dropwise to a stirred solution of 3,4-dimethoxybenzaldehyde (10.0 g, 60.1 mmol) in dry chloroform (80 mL) under argon at room temperature. The mixture was heated to 60°C for 6 h, and after cooling, it was concentrated in vacuo. The residue was washed with chloroform and evaporated under reduced pressure. Crystallization from MeOH afforded bromoveratraldehyde 9c (13.1 g, 89%) as a white powder: mp 147-148°C (lit. 149-151°C) References [1] Org. Proc. Res. Dev., 3 (6), 425-431 (1999)
|