facts:
*) CO2 evolves during this reaction
*) Cu precipitates
if we only look at electrons, not configuration:
we start with:
R-COO-Cu-COO-R (I)
minus CO2:
R-Cu-R (II)
now if metallic copper precipitates we end up with
R-R (III) (=dimeric, disgusting crap)
i'm pretty sure that precipitated copper means ugly side product,
what we really want is (II), which when hydrolised with H2O gives:
2R-H + Cu(OH)2
i have no idea what (II) looks like. i can imagine the beginning of
the reaction, which i think looks like this:
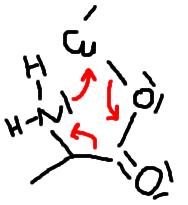
this would make sense, considering that all Ns and Os interact with
the empty d-orbitals of the Cu.
but what is the end product?
surely not this:

(yes i know, you can't write structures like this)
if nobody disagrees, i'll just continue believing that
the world is flat...
