Found a few boxes of lithium polymer batteries at a cell phone refurbishing plant, bought them for really cheap. Does it matter if the battery id charged or not for it to work for a birch?
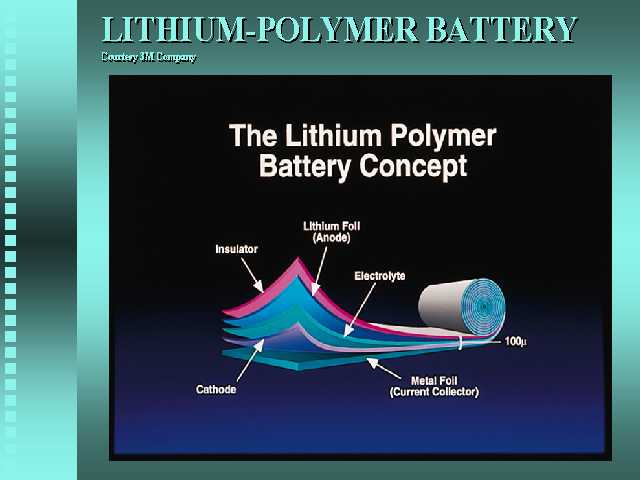
Some random info of lith polymer:
The Lithium Polymer battery relies on thin-film technology, with composite films that are only 100 microns thick. It’s a solid state battery that can be wound and shaped to suite the application. It uses a plastic electrolyte. 3M expects that a typical EV battery pack would weigh on the order of 500 pounds (224 kg), which could provide as much as 45 kW-h of energy. In comparison, EV1’s lead-acid battery pack weighs over 1000 pounds (480 kg) and provides 16 kW-h of energy. So we have the potential of storing nearly 3 times the energy with half the mass of today’s lead-acid batteries.
Cy
We are the people that your parents warned you about.