For Nicodem:4-Hydroxy-1-[2-(4-hydroxyphenoxy)ethyl]-4-(4-methylbenzyl)piperidine:
A Novel, Potent, and Selective NR1/2B NMDA Receptor AntagonistZ.-L. Zhou, S.X. Cai, E.R. Whittemore, C.S. Konkoy, S.A. Espitia, M.Tran, D.M. Rock, L.L. Coughenour,
J.E. Hawkinson, P.A. Boxer, C.F. Bigge, L.D. Wise, E. Weber, R.M. Woodward, and J.F.W. KeanaJ. Med. Chem. 42, 2993-3000 (1999) 


 Abstract
AbstractA structure-based search and screen of our compound library identified N-(2-phenoxyethyl)-4-benzylpiperidine (
8) as a novel N-methyl-D-aspartate (NMDA) receptor antagonist that has high selectivity for the NR1/2B subunit combination (IC
50 = 0.63 µM). We report on the optimization of this lead compound in terms of potency, side effect liability, and in vivo activity. Potency was assayed by electrical recordings in
Xenopus oocytes expressing cloned rat NMDA receptors. Side effect liability was assessed by measuring affinity for 1-adrenergic receptors and inhibition of neuronal K
+ channels. Central bioavailability was gauged indirectly by determining anticonvulsant activity in a mouse maximal electroshock (MES) assay. Making progressive modifications to
8, a hydroxyl substituent on the phenyl ring para to the oxyethyl tether (
10a) resulted in a ~25-fold increase in NR1A/2B potency (IC
50 = 0.025 µM). p-Methyl substitution on the benzyl ring (
10b) produced a ~3-fold increase in MES activity (ED
50 = 0.7 mg/kg iv). Introduction of a second hydroxyl group into the C-4 position on the piperidine ring (
10e) resulted in a substantial decrease in affinity for 1 receptors and reduction in inhibition of K
+ channels with only a modest decrease in NR1A/2B and MES potencies. Among the compounds described,
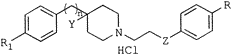
10e (4-hydroxy-N-[2-(4-hydroxyphenoxy)ethyl]-4-(4-methylbenzyl)piperidine, Co 101244/PD 174494) had the optimum pharmacological profile and was selected for further biological evaluation.