Anyone with access to Organic Reactions Online? They offer the first page of a whole lot of comprehensive review articles, just like the ones below...
The table of contents for the articles look really enticing, don't you think?  Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing AgentsEllen W. Baxter, Allen B. Reitz
Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing AgentsEllen W. Baxter, Allen B. Reitz,
Organic Reactions (2002), John Wiley and Sons, Inc.DOI:
10.1002/0471264180.or059.01
Abstract
1. Introduction
2. Mechanism and Stereochemistry
3. Scope and Limitations: The Reducing Agent
4. Scope and Limitations: The Carbonyl Component
5. Scope and Limitations: The Amine Component
6. Intramolecular Reductive Aminations
7. Side Reactions
8. Failed Reactions
9. Reductive Aminations on a Solid Support
10. Tandem Reactions
11. Comparison with Other Methods
12. Experimental Conditions
13. Experimental Procedures
14. Tabular Survey
15. Acknowledgments
ReferencesKeywords: organic reaction(s); organic synthesis; reaction(s); synthesis; reductive amination; condensation; alkylation; reductive alkylation; reduction; amination; carbonyl; amine; reducing agent(s); borohydride; borane; carbon-nitrogen bond(s); CN bond(s)AbstractReductive amination is an important tool for synthetic organic chemists in the construction of carbon-nitrogen bonds. This reaction, also termed reductive alkylation, involves condensation of an aldehyde or ketone with an amine in the presence of a reducing agent. A wide variety of substrates can be used including aliphatic and aromatic aldehydes and ketones, and even benzophenones. A range of amines from ammonia to aromatic amines, including those with electron-withdrawing substituents, can be employed. For particularly sluggish reactions, such as those involving weakly electrophilic carbonyl groups, poorly nucleophilic amines, or sterically congested reactive centers, additives such as molecular sieves or Lewis acids are often useful.
This chapter focuses on those conditions in which the carbonyl component, amine, and reducing reagent react in the same vessel. This review is restricted to reductive aminations using borohydride and borane reducing agents. This chapter concentrates on reductive amination chemistry mediated by borohydride and other boron-containing reducing agents from 1971, the year when sodium cyanoborohydride was introduced, through the middle of 1999. In addition to reductive aminations of aldehyde and ketone substrates, reactions of related structures including acetals, aminals, ketals, carboxylic acids, nitriles, and dicarbonyls that form a nitrogen-containing ring are reviewed. Intramolecular processes in which the substrate contains both the carbonyl and amine moieties are described. The intramolecular variant is a useful method for preparing cyclic amines. All of the various boron-containing hydride sources in reductive aminations, including labeled metal hydrides, are reviewed. Instances of reductive aminations that failed are described. Applications of this method to a solid support in parallel synthesis in combinatorial chemistry as well as reductive aminations that proceed in tandem with a second reaction such as reductive lactamizations are discussed.
1. IntroductionReductive amination is an important tool for synthetic organic chemists in the construction of carbon-nitrogen bonds. This reaction, also termed reductive alkylation, involves condensation of an aldehyde or ketone with an amine in the presence of a reducing agent as illustrated in Eq. 1. A wide variety of substrates
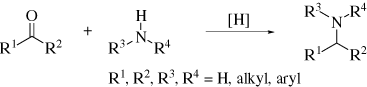
can be used including aliphatic aldehydes and ketones, aromatic aldehydes and ketones, and even benzophenones. Further, a range of amines from ammonia to aromatic amines, including those with electron-withdrawing substituents, can be employed. For particularly sluggish reactions, such as those involving weakly electrophilic carbonyl groups, poorly nucleophilic amines, or sterically congested reactive centers, additives such as molecular sieves or Lewis acids are often useful.
Reductive aminations have been reviewed on numerous occasions, (1-17) and this chapter focuses on those conditions in which the carbonyl component, amine, and reducing agent react in the same vessel. The reduction of a preformed, isolated species such as an imine or oxime is not covered. This review is also restricted to reductive aminations using borohydride and borane reducing agents. Reactions carried out with other metal hydrides or inorganic reducing agents in addition to catalytic hydrogenations, Leuckart conditions, and enzymatic reductive aminations are not included. A review summarizing reductive alkylation of proteins has been published recently, (18) and these substrates are not covered here. This chapter concentrates on reductive amination chemistry mediated by borohydride and other boron-containing reducing agents from 1971, the year when sodium cyanoborohydride was introduced by Borch and coworkers, (19) through the middle of 1999. Although we have been as inclusive as possible, there are almost certainly additional references that we inadvertently missed. We apologize in advance to those authors who do not see their own contributions cited here.
In addition to reductive aminations of aldehyde and ketone substrates, we review reactions of related structures including acetals, aminals, ketals, carboxylic acids, and nitriles as well as dicarbonyl substrates that form a nitrogen-containing ring. Intramolecular processes in which the substrate contains both the carbonyl and amine moieties are described. In these reactions, one of the components is typically masked, and reductive amination occurs upon deprotection. The intramolecular variant is a useful method for preparing cyclic amines.
While sodium cyanoborohydride is the best known hydride reagent for reductive alkylations, sodium borohydride is often used as well. (20) Sodium triacetoxyborohydride is now widely used because it is nontoxic and generally does not reduce the carbonyl group prior to imine formation. (21) Amine boranes such as borane-pyridine are also employed in reductive aminations. (22) We review all of the various boron-containing hydride sources in reductive aminations in this chapter, including labeled metal hydrides such as sodium cyanoborodeuteride.
Instances where reductive aminations fail are described, including cases when reaction is not observed and also where side products appear, such as alcohols and bis-alkylated amines.
Finally, we discuss the application of this method to a solid support in parallel synthesis and combinatorial chemistry as well as reductive aminations that proceed in tandem with a second reaction such as in reductive lactamizations.
The Tabular Survey at the end of the chapter includes thousands of specific reactions and applications for reductive aminations, including sections on aldehydes, ketones, dicarbonyl substrates, tricarbonyl substrates, carboxylic acids, nitriles, intramolecular reductive aminations, reductive lactamizations, and Michael-type additions and reductive aminations.
Dioxirane Epoxidation of AlkenesWaldemar Adam, Chantu R. Saha-Möller, Cong-Gui ZhaoOrganic Reactions (2002) John Wiley & Sons, Inc.DOI:
10.1002/0471264180.or061.02
Abstract
1. Introduction
2. Mechanism
3. Scope and Limitations
4. Comparison with Other Methods
5. Experimental Conditions
6. Experimental Procedures
7. Tabular Survey
8. Acknowledgments
ReferencesKeywords: dioxirane; epoxidation; alkenes; unfunctionalized alkenes; electron-rich; electron poor; electron donor; electron acceptor; chemoselectivity; regioselectivity; diastereoselectivity; entioselectivity; scope; limitations; oxidation; solvents; temperature; neutral conditions; basic conditions; homogeneous media; comparison of methods; experimental conditions; experimental procedures; tabular surveyAbstractAn ideal oxidant should be highly reactive, selective, and environmentally benign. It should transform a broad range of substrates with diverse functional groups, preferably under catalytic conditions, and be readily generated from commercially available and economical starting materials. Of course, such an ideal oxidant has not yet been invented; however, the dioxiranes, which have risen to prominence during the past few decades, appear to fulfill these requirements in many respects. These three membered ring cyclic peroxides are very efficient in oxygen transfer, yet very mild toward the substrate and product. They exhibit chemo-, regio-, diastereo-, and enantioselectivities, act catalytically, and can be readily prepared from a suitable ketone (for example, acetone) and potassium monoperoxysulfate ( 2KHSO5 · K2SO4 · Caroate®, Oxone®, or Curox®), which are low-cost commercial bulk chemicals. Throughout the text KHSO5 is used to specify this oxygen source, rather than refer to one of the commercial trade names.
Isolated dioxiranes (as solutions in the parent ketones) perform oxidation under strictly neutral conditions so that many elusive oxyfunctionalized products have been successfully prepared in this way for the first time. Epoxidations, heteroatom oxidations, and X-H insertions constitute the most investigated oxidations by dioxiranes. An overview of these transformations is displayed in a rosette scheme. These preparatively useful oxidations have been extensively reviewed during the last decade in view of their importance in synthetic chemistry.
This chapter deals mainly with the epoxidation of carbon-carbon double bonds [ bonds in simple alkenes and with these electron donors (ED), electron acceptors (EA), and with both ED and EA] with either isolated or in situ generated dioxiranes. In view of the vast amount of material on alkene oxidation, the epoxidation of the double bonds in cumulenes (allenes, acetylenes) and arenes is covered in a separate chapter, together with the oxidation of heteroatom functionalities (nonbonding electron pairs), X-H insertions ( bonds) and transition-metal complexes.