Cobalt(II) catalyzed tosylation of alcohols with p-toluenesulfonic acid Subbarayan Velusamy, J. S. Kiran Kumar and T. Punniyamurthy*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India Received 23 July 2003; revised 7 October 2003; accepted 17 October 2003
Tetrahedron Letters 45 (2004) 203–205
DOI:
10.1016/j.tetlet.2003.10.106
Full-text PDF:

Abstract—Cobalt(II) chloride hexahydrate (CoCl
2.6H
2O) has been found to catalyze the tosylation of both aliphatic and aromatic alcohols with p-toluenesulfonic acid (p-TsOH) in high yields in 1,2-dichloroethane under re?ux (ca. 80 °C). In the case of aliphatic alcohols, secondary alcohols undergo tosylation chemoselectively in the presence of primary hydroxy groups.
Keywords: tosylation; alcohol; p-toluenesulfonic acid; catalyst; cobalt(II) chloride hexahydrate.
The tosylation of alcohols is a frequently used functional transformation in organic chemistry.
1 Typically, the moisture sensitive and very reactive sulfonyl chloride or anhydride are usually employed as the tosylating agents in the presence of bases.
2 Sulfonic acids are also used as tosylating agents but expensive alkylating agents such as trialkyl orthoformate, alkyl ethers or esters are needed instead of the alcohol.
3 Recently, organic base adducts of sulfonyls such as 1-phenylsulfonyl benzotriazole and aryl sulfonyl methylimidazolium salts have been employed for sulfonate synthesis, however, significant amounts of by-products in the form of total dissolved salts are generated.
4 More recently, Fe
3+ montmorillonite clay has been reported to catalyze the tosylation of alcohols with p-TsOH.
5 This method works well with aliphatic alcohols but aromatic alcohols give rearranged or polymeric products. Herein, we wish to report a simple and effcient method for the direct tosylation of both aliphatic and aromatic alcohols with 1 equiv of p-TsOH in the presence of CoCl
2.6H
2O in high yields (Scheme 1). This protocol also functions in the absence of additives and the removal of water is not warranted. In contrast to the Fe
3+–montmorillonite catalyzed process,
5 this methodology chemoselectively catalyzes the tosylation of aliphatic secondary alcohols in the presence of primary hydroxy groups in high yields.
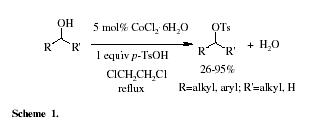
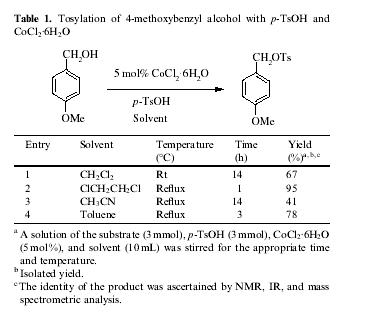
In the presence of a catalytic amount of CoCl
2.6H
2O, the tosylation of 4-methoxylbenzyl alcohol was ?rst examined with p-TsOH (Table 1). Toluene, CH
2Cl
2,CH
3CN, and ClCH
2CH
2Cl were employed as the solvents. As expected, the reaction took place and afforded 4-methoxybenzyl tosylate in good yields when the reaction mixtures were allowed to stir in the presence of 5 mol% CoCl
2.6H
2O. Among the solvents screened, ClCH
2CH
2Cl was found to be suitable for this protocol and the highest yield of 95% was obtained under re?ux (ca. 80 °C). The reactions in CH
2Cl
2, CH
3CN, and toluene were less effective and afforded the tosylate in 41–78% yields. Control reactions without CoCl
2.6H
2O under the same reaction conditions showed no reaction.
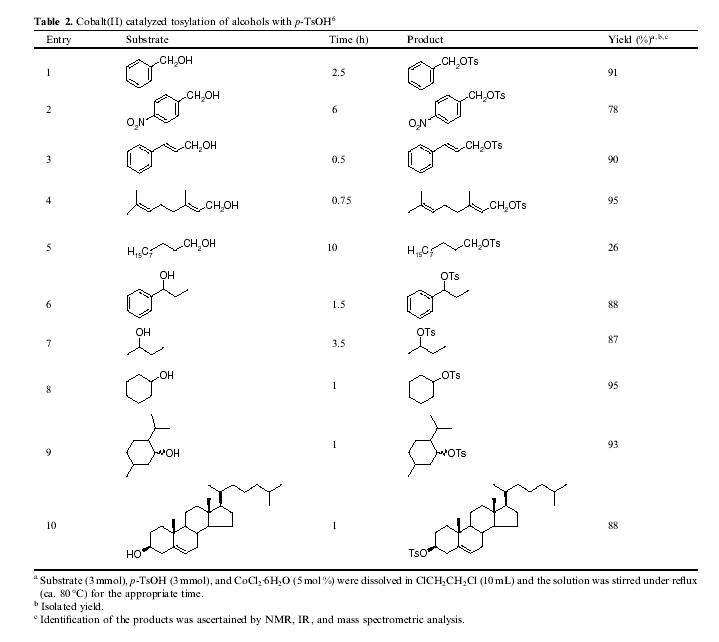
The reactions of a series of other aromatic and aliphatic alcohols were next pursued with p-TsOH in ClCH
2CH
2Cl as above (Table 2, entries 1–10).
6 Benzylic alcohols, benzyl-, 4-nitrobenzyl, and 1-phenylpropyl alcohol, were converted to the corresponding tosylates in 78–91% yields. Similarly, allylic alcohols, cinnamyl alcohol, and geraniol, underwent tosylation to afford the corresponding tosylates in 90–95% yields. In the case of the aliphatic alcohols, secondary alcohols were tosylated much faster compared to primary hydroxy groups. For example, 2-butanol, cyclohexanol, menthol, and cholesterol were transformed into the corresponding tosylates in 87–95% yields within 3.5 h, whereas decanol was less reactive and afforded decyl tosylate in only 26% yield after 10 h. When a mixture of cyclohexanol and n-hexanol was allowed to react with 0.5 equiv p-TsOH for 1 h, cyclohexanol underwent tosylation chemoselectively in 95% yield along with a trace of n-hexyl tosylate (Scheme 2). These studies clearly reveal that this methodology can be applied for the chemoselective direct tosylation of aliphatic secondary alcohols in the presence of primary hydroxy groups. Furthermore, when the tosylation of phenol and b-naphthol was examined, no reaction was observed and the starting materials were recovered intact.
In conclusion, the present methodology describes a simple procedure for the direct tosylation of alcohols with p-TsOH in the presence of the inexpensive CoCl
2.6H
2O in high yields. It functions in the absence of additives and generates water as the only by-product. In the case of aliphatic alcohols, secondary alcohols are transformed to the corresponding tosylates chemoselectively in the presence of the primary hydroxy groups.
 Acknowledgements
Acknowledgements This work was supported by the Department of Science and Technology (sanction no. SR/S1/OC-092002), New Delhi and by the Council of Scienti?c and Industrial Research (sanction no. 01(1804)/02/EMR-II), New Delhi.
References and Notes 1. Greene, T. W.; Wuts, P. G. M.
Protective Groups in Organic Synthesis. 3rd ed. Wiley: New York, 1999.
2. (a) Kabalka, G. W.; Varma, M.; Varma, R. S.; Srivastava, P. C.; Knapp, F. F., Jr.
J. Org. Chem. (1986) 51, 2386;
(b) Yoshida, Y.; Sakakura, Y.; Aso, N.; Okada, S.; Tanabe, Y.
Tetrahedron (1999) 55, 2183;
(c) Hartung, J.; Hunig, S.; Kneuer, R.; Schwarz, M.; Wenner, H.
Synthesis (1997) 1433.
3. Nitta, Y.; Arakawa, Y.
Chem. Pharm. Bull. (1985) 33, 1380.
4. (a) O'Connell, J. F.; Rapoport, H.
J. Org. Chem. (1992) 57, 4775;
(b) Katritzky, A. R.; Zhang, G.; Wu, J.
Synth. Commun. (1994) 24, 205.
5. Choudary, B. M.; Chowdari, N. S.; Kantam, M. L.
Tetrahedron (2000) 56, 7291.
6. Alcohol (3 mmol), p-TsOH (3 mmol) and CoCl
2.6H
2O (5 mol %) were dissolved in ClCH
2CH
2Cl (10 mL) and the solution was stirred under re?ux (ca. 80 °C) for the appropriate time (see Table 2). The reaction mixture was then allowed to cool to ambient temperature and diethyl ether (50 mL) was added. The catalyst was removed by filtration and the filtrate was washed successively with saturated NaHCO
3 solution (3 x 10 mL), brine (2 x 10 mL), and water (1 x 10 mL). Drying (Na
2SO
4) and evaporation of the solvent on a rotary evaporator afforded a residue, which was passed through a short pad of silica gel using a mixture of ethyl acetate and hexane as eluent to afford the analytically pure tosylate.