A New Vanillin SynthesisJ. Am. Chem. Soc. 2107-2108 (1934)SummaryA method for the synthesis of vanillin has been advanced which depends upon the Fries rearrangement and the oxidation of an acetophenone type linkage to a substituted glyoxylic acid.
 Introduction
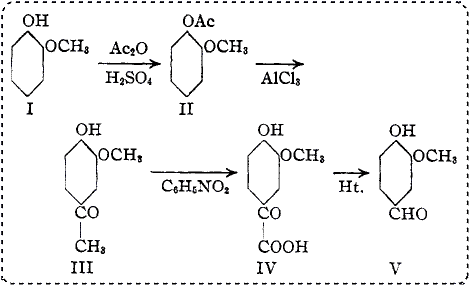
IntroductionSyntheses of vanillin from guaiacol have depended largely upon a direct addition of a carbon atom to the 4-position in guaiacol. Guaiacol, possessing all the characteristics of a phenol, often produces large amounts of resin polymers when this type of reaction is used.
1 In an attempt to eliminate the troublesome resin formation, the following synthesis of vanillin was devised. The synthesis depends upon the rearrangement of the acetyl group in guaiacol acetate to the 4-position, and subsequent oxidation of this group to form a substituted glyoxylic acid. The rearrangement follows the prediction of Fries and others.
2 Guaiacol (I) is esterified with acetic anhydride in the presence of a trace of sulfuric acid to form guaiacol acetate (II). Guaiacol acetate treated with anhydrous aluminum chloride at low temperature rearranges to apocynin. The acyl group takes the position para to the hydroxy group rather than to the methoxy group. No 3-hydroxy-4-methoxyacetophenone seems to be formed.
3 If the addition of aluminum chloride is made too rapidly and the temperature rises, small amounts of acetyl chloride are formed, which seems to support the assumption that the Fries rearrangement depends upon an intermediate formation of the acid chloride.
Apocynin (III) when treated with a quantity of nitrobenzene, theoretically required for oxidation to the glyoxylic acid, in a strongly alkaline solution, forms vanilloylformic acid (IV). This acid is much more stable toward acid and alkaline reagents than the corresponding 4-hydroxy-3-methoxyphenylglycolic acid, which tends to dehydrate and form resinous products.
The decomposition of vanilloylformic acid
4 should be done in a solvent to prevent the formation of resin, dehydrovanillin and vanillic acid. Heating in boiling aniline
5 prevents the formation of dehydrovanillin and vanillic acid by favoring the formation of the Schiff base. Dimethylaniline reacts with vanillin to form (4-hydroxy-3-methoxyphenyl)-bis-(4-dimethylaminophenyl)-methane. However, dimethyl-p-toluidine causes the decomposition to become almost quantitative for vanillin. This solvent affords an excellent medium.
Experimental PartGuaiacol Acetate 62 grams of guaiacol (0.5 mole), and 56 g. of acetic anhydride (0.55 mole) were mixed together. Coned. sulfuric acid (0.5 cc.) was added and the mixture well shaken. The reaction mixture developed heat immediately and was kept at 100° for three hours. The mixture was then cooled and poured into a small amount of water and carefully neutralized with sodium carbonate. Care is necessary in neutralizing because of the ease of hydrolysis of the ester. No excess alkali must be added and the solution kept cold. Vacuum distillation of the neutral oil gave 81 g of guaiacol acetate, bp 123-124°C at 13 mm.; yield 98%.
Apocynin75 grams (0.45 mole) of guaiacol acetate was placed in a flask fitted with an efficient stirrer Vol. 56 and cooled by an ice-salt bath. At 0°C, 77g (0.585 mole) of finely powdered anhydrous aluminum chloride was added in small portions, keeping the temperature below 5°C and stirring so that the mixture did not lump. After the addition of the aluminum chloride the mixture was allowed to stand for twenty-four hours. The thick mass, usually dark red, was poured over ice and taken up in ether. The ether solution was carefully neutralized and dried over anhydrous sodium sulfate. The ether was distilled off and the residual oil vacuum distilled. Small amounts of guaiacol were recovered with guaiacol acetate; 38 g of apocynin was obtained, bp 160-170°C at 13 mm.; 34 g of guaiacol acetate was recovered for use again; yield of apocynin 97%; conversion, 50%; mp 115°C. If a strong solution of sodium carbonate or potassium carbonate is used to neutralize the acid ether solution of apocynin-guaiacol acetate, a salt of apocynin is formed which is precipitated and which is also slightly soluble in water. This may be the source of some loss. It is possible to obtain apocynin of high purity from these salts.
Vanilloylformic Acid35g (0.21 mole) of apocynin, 27 g. of nitrobenzene (0.22 mole) and 25 g. of sodium hydroxide (0.62 mole) were mixed in 75 cc. of water and kept at 100° for twenty-four hours. On cooling, none of the sodium salt of apocynin crystallized out. The aniline and a small mount of nitrobenzene were removed by steam distillation. When cold the solution was acidified, decolorized with charcoal while hot and the acid crystallized. As the acid is quite soluble in water its solution may be repeatedly concentrated to obtain all the acid; crude yield 34 g., 85%, mp 133°C.
Vanillin 25 grams of vanilloylformic acid and 25g of dimethyl-p-toluidine were heated at 170°C until the evolution of carbon dioxide had ceased. The mixture was taken up in ether and washed with dilute hydrochloric acid until the amine was recovered, The ether solution was neutralized and dried over anhydrous sodium sulfate. On distillation of the ether 37.5g of crude vanillin was obtained; yield, 98%; recrystallized from ligroin, mp 80°C.
References (1) Ullmann,
"Enzyklopädie der technischen Chemie," Vol. VIII, p. 815, a review of existing methods of manufacture.
(2) Fries and Pfaffendorf,
Ber., 43, 214 (1910); v. Auwers and Risse,
Ber., 64, 2216 (1931); Cox,
JACS 52, 352 (1930).
(3) Otto,
Ber., 24, 2869 (1891).
(4) Guyot and Gry,
Compt. Rend., 149, 930 (1909);
Bull. soc. chim., 141 7, 912 (1910).
(5) Gassmann,
Compt. rend., 124, 38 (1897).