This review is about precursors to 2C-T-x compounds. The linked thread to this review is
Post 433179
(catastrophe: "Aralkyl halides to Thiols", Chemistry Discourse), you can post comment on it in this thread.
The 2CT family is really interesting, those compounds are usually nice, and relatively potent. They are psychedelics active in the 10-100 mg range, with duration from 3 to 18 hours, depending solely on the 4-alkyl group. All those compounds use the same global precursors and the same synthetic scheme, so if you can synthetise one, you can synthetise the others too.
For further information on those chemicals, see Pihkal #39 to #49.
Keywords: Thiols, alkylthio, dimethoxy thiophenol, mercaptans, thiourea, 2c-t, 2ct2, 2-ct-2, 2c-t-2, 2ct7, 2-ct-7, 2c-t-7, 2ct4, 2-ct-4, 2c-t-4, 2c-t-21, 2c-t-22.
1. Summary:I will use 2ct2 as example, but the S-ethyl group can bee exchanged with other ones, of course (altough with some limitation).
I assumed the target compound to be the 4-alkylthio-dimethoxybenzaldehyde, because the nitrostyrene route is by far the more used way to make phenetylamines, altough there are many other ethylamine side chain synthesis (some start from the aldehyde, like the Barbier reaction or from mandelonitrile, other start from the straight phenyl, those are cited below).
There are two main routes to go to the final aldehyde
Z:
The first start with 1,4-dimethoxybenzene
A, transform it by various ways into a 2-alkylthio-dimethoxybenzene
D and then formylate it to
Z.
The second start with good ol' 2,5-dimethoxybenzaldehyde and transform it to
Z.
Another method is from benzoquinone, by reaction with thiourea or sodium thiosulfate, followed by alkylation of the thiol, then methylation of the phenols, to yield the compound
D.
It should be stressed than the compound
D is also a very useful precursor if other synthetic methods to generate the N-ethyl side chain are used. Those methods include chloromethylation, arynation, mannich base, mercuration, lithiation, alkylation with a protected amine group (phthalimidoethyl bromide alkylation for instance).
This is what we have so far, I used the well known 2-CT-2 molecule as example.
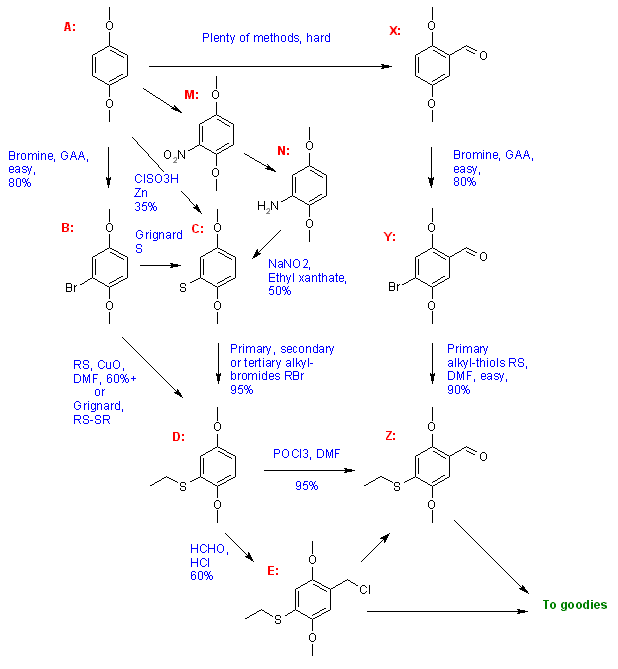
2. Presentation of the two routes2.1 Precursors overview:1,4-Dimethoxybenzene
A is not watched, and not expensive. It can bee prepared in a street situation from hydroquinone, by methylation with CH3I. Alternatively it can be prepared from p-methoxy-phenol.
2,5-Dimethoxybenzaldehyde
X is watched, and relatively expensive. It can bee prepared in a street situation from hydroquinone through p-methoxy-phenol. It can be prepared from dimethoxybenzene by various ways, most of them are hard (grignard) and/or use possibly watched chemical (dichloromethyl methyl ether) and/or dangerous chemical (HCN) and/or have crappy yield (chloromethylation). The most prefered way on the street should be azomethinic reaction.
2.2 General pathway overview:2.2.1 From 1,4-dimethoxybenzene2.2.1.a Shulgin's wayGood doctor Shulgin use the
A -> C -> D -> Z route.
The
A -> C reaction is made with a total yield of 35%. It involve the reaction of dimethoxybenzene with chlorosulfonic acid, to yield 2,5-dimethoxybenzenesulfonyl chloride, then he reduct that with Zn dust to 2,5-dimethoxythiophenol in 35% yield. The reaction look not hard and is odorless according to Yellium. Its a good way but chlorosulfonic acid is a very corrosive material, and acquiring it should be the biggest problem of that way (altough it is used to make detergents).
Shulgin then alkylate the thiophenol
C such obtained to
D in yield of 95%. Various alkylation of the thiophenol are found in Pihkal #39 to #49. The alkylation proceed in good yield for both primary and secondary alkyl halide.
Shulgin does a formylation step from the various substituted-benzenes
D to corresponding benzaldehydes
E. He use the well known (and watched) phosphoryl chloride to do those formylation (with NMF), or dichloromethyl methyl ether (2C-T).
Yield are good (75-95%) for most compounds, except 2c-t-9 and 2c-t-13. The detailed syntheses are in PiHKAL of course. It is most probable than NMF could be replaced by DMF.
Please note that those alkylthio-dimethoxybenzene are more activated than straight 1,4-dimethoxybenzene. The POCl3 procedure does not work on 1,4 dimethoxybenzene and the dichloromethyl methyl ether procedure has got only moderate yield (50%). Here we saw good yields for both procedure on the alkylthio-2,5-dimethoxybenzenes.
To go from
E to the final amines, Sacha synthetise the nitrostyrenes and reduct with LAH. Most bee will surely use Beaker/Barium reduction pathway instead of LAH.
This was the classic route. It work well but its chems are a bit hard to get, so we have to look at alternative syntheses.
2.2.1.b Czechoslovak/Xanthate route.This route was used by some Czechoslovak scientists. It is a completely different and very interesting route, on the picture it is the
A -> M -> N -> C -> D -> E -> To Goodies route.
The
A -> M conversion is just a standard nitration. but proper conditions are required to avoid dinitration. I have no ref about that nitration.
The
M -> N reduction can be done by multiple reagents. I had a ref about that particular transformation, but it was small scale and not optimized.
The direct conversion
A -> N is possible, it involve a reaction of dimethoxybenzene with sodium in liquid ammonia. This ammonolyse is a competiting reaction in the aryne route to phenylacetonitriles which involve treating dimethoxybenzene with acetonitrile in liquid ammonia. Proper conditions favorise the amination or the alkylation, and the yields are usually good.
The amine
N must be diazotised before treatment with potassium ethyl xanthate. The diazotation step is made easilly with NaNO2 in cold. The mixture is then poured into a solution of potassium ethyl xanthate in warm water, after reaction the adduct is hydrolised. See
Post 423119
(Chimimanie: "Synthesis of 2-Chloromethyl-5-alkylthio-DMB", Methods Discourse) for a description of the process. The yield is 50% from the 2,5-dimethoxy-aniline
N to the thiophenol
C according to the text, but no experimental data is given for our particular compound.
The alkylation of
C to
D follow standard procedures. It pose no problem and yield are excellent with pentyl and heptyl bromide. There is no need for the sodium ethoxide they used, shulgin method with KOH will work great too.
Here is the most interesting part of the Czechoslovak article, they chloromethylate the alkylthio compounds
D to the chloromethyl derivatives
E. The yields are moderate (60%).
The chloromethyl group is a useful synthon for conversion to the aldehyde
E -> Zor to the phenylacetonitrile derivative (not represented). The NaCN swap in DMSO work well (70-80%).
Finally the czechoslovak team reduce those phenylacetonitriles to the corresponding amines with LAH. Sadly, and this is the big stumbling block in the second part of this synthesis, there are no good LiAlH4 replacement for the reduction of phenylacetonitriles. Altough NaBH4 with nickel or cobalt co-reagent could be used, yields are not great or the reductions are done with a too big NaBH4 consumption.

We need OTC nitriles reduction.
Research in alternative reduction of phenylacetonitriles are a must! I call for the enterprising bees...EDIT: it seem that the Zn/Ni couple may be a good candidate 2.2.1.x Lithiation/disulfide reactionYet another way is the direct conversion of 1,4-dimethoxybenzene
A to the alkylthio analog
D. It is elegant but hardcore chemistry: it involve the lithiation of dimethoxybenzene and then reaction with a disulfide. Shulgin use that route for 2-CT-9.
Also, grignard reaction of bromo-dimethoxybenzene with sulfur yield the thiophenol
Post 437082
(Rhodium: "Some precursors we haven't mentioned yet", Chemistry Discourse)2.2.2 From 2,5-dimethoxybenzaldehydeThis is the
X -> Y -> Z route.
The hardest part in this route is to acquire 2,5-DMB
X, when you have it you are close to your goal
Z.
This route involve the bromination of 2,5-DMB to its 4-bromo-analogue
Y. The standard bromination in GAA
https://www.thevespiary.org/rhodium/Rhodium/chemistry/4-alkylthio-25-dmb.html
, yield a 4:1 ratio of 4-bromo-2,5-dimethoxy-benzaldehyde and 2-bromo-3,6-dimethoxybenzaldehyde. After recrystallisation the 4-bromo-isomer is obtained in ~50% yield. But a 87% yield of the 4 isomer is obtained by this procedure:
https://www.thevespiary.org/rhodium/Rhodium/chemistry/bromodimethoxybenzaldehyde.html
. There are also preparations of this brominated aldehyde starting from bromo-dimethoxybenzene through Gatterman formylation

or from dibromo-dimethoxybenzene through grinard reaction. See
Post 414337
(Chimimanie: "This describe a grignard on ...", Novel Discourse).
Two papers desribe the nucleophilic displacement of the bromine atom by a thiol, after analyse of the two references
Post 436683
(Chimimanie: "Little analysis of the dimethoxybenzaldehyde refs", Chemistry Discourse) we can conclude that this reaction is especially convenient, because:
-It only involve stirring the primary alkyl mercaptan in DMF with the brominated dimethoxybenzaldehyde, at room temperature, with K2CO3 as a base.
-The yields are good.
-It can bee done in a closed RBF, without propagating noxious odors anymore.
-Nitrogen is not necessary, according to the spanish article at Rhodium's.
After reaction, the vessel is opened, the content poured in water, filtered and recrystallised to afford the aldehyde
Z.
Contrary to Shulgin's way where the 4-alkyl group is made by alkylating a thiophenol, with an alkyl bromide, here we have to start from an appropriate alkyl mercaptan, and react it with an aryl bromide.
The mercaptans must be prepared before reaction, from the alcohols through the bromide. Methods for preparating those mercaptans are shown below.
The most important limitation of this method is that it is only useful for primary alkyl mercaptans

, altough by debenzylation of the 4-benzylthio aldehyde the thiophenol can be obtained and then alkylated with secondary or tertiary alkyl bromides to yield the missing aldehydes
Z.
Here are some ref on benzyl mercaptan synthesis: (If a kind bee can post it in the linked thread of the digest?)
JACS 68 1946 2104 benzyl alcohol -> benzyl mercaptan w/ thiourea
Rec trav chim Pays-Bas 67 1948 884,892,893 and
Rec trav chim Pays-Bas 51 1932 290: benzyl chloride -> benzyl mercaptan w/ thiourea
2.2.3 Other ways:2.2.3.a From vanillin to thiovanillin then 2-methoxylation. Alternatively from syringaldehyde to form mescalin thio analogues (4-TM, 4-TE). Look nice for syringaldehyde! see
Post 259352
(Rhodium: "Re: thiovanillin", Novel Discourse) and
Post 122786
(dormouse: "vanillin as a starting material for 2-CT's -rev drone", Serious Chemistry).
2.2.3.b From benzoquinone dimethyl acetal, by reaction with thio compounds. This look interesting, it should be further researched. See compounds
2e,
2f and
2g in
Post 473897 (missing)
(Lego: "2,5-Dimethoxyprecursors from benzene or anisole", Chemistry Discourse) for example.
2.2.3.c From benzoquinone by reaction with thiosulfate then S-alkylation and O-methylation, then chloromethylation or formylation. or from straight reaction of benzoquinone with a mercaptan compound and from there the same reactions. See 2C-T PiHKAL #39 and
Post 360999
(Rhodium: "2,5-Dihydroxythiophenol and alkoxy derivatives", Novel Discourse).
2.2.3.d From 1,4-dimethoxybenzene with S2Cl2 and a lewis acid (ZnCl2...), then reduction (Na2S) like
Post 207554
(Antoncho: "An easy OTC 2,5-diMeO-phenylmercaptan", Novel Discourse) suggested.
....
Synthesis of starting alkyl mercaptan compounds (RS):A practical way to synthetise these compounds is from an alkyl bromide followed by treatment with thiourea and basic hydrolysis. The alkyl bromide can be generated
in situ with KBr/H2SO4 and directly treated with thiourea.
Beware those compounds have low boiling point (methanethiol is a gas, ethanethiol bp 35°C, n-propanethiol bp 67°C) and they stench.
Ethanethiol, ethyl mercaptan:S-Ethylthiourea hydrobromide.A mixture of 150 g. of powdered thiourea (1.97 moles) (Note 1), 250 g. of ethyl bromide (2.29 moles), and 200 ml. of absolute ethanol is placed in a 1-l. round-bottomed flask equipped with an efficient condenser. The mixture is warmed on a water bath (bath temperature 55–65°) for 3 hours, with occasional shaking. During this time all the thiourea dissolves. The reflux condenser is replaced by one set for downward distillation, and the ethanol and excess ethyl bromide are removed under the vacuum of a water pump. During the distillation, the temperature of the bath is slowly raised to the boiling point (Note 2). The residual oil is poured into a 500-ml. beaker and allowed to crystallize (Note 3). The solid is pulverized and dried in a desiccator (Note 4) and (Note 5). The yield is 340–360 g. (93–99%).
Basic hydrolysis of this salt yield the corresponding thiolate.
ref: http://www.orgsyn.org/orgsyn/prep.asp?prep=cv3p0440
Other examples of mercaptan preparation:
n-dodecyl mercaptan
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv3p0363
2-Cyanoethanethiol
http://www.orgsyn.org/orgsyn/prep.asp?prep=v77p0186
2-Furanmethanethiol
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv4p0491
1,2-Ethanedithiol
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv4p0401%5Bb
]
The needed bromides can be synthetised from H2SO4 and KBr, to generate HBr
in situ.
There are various synth of alkyl bromides from the alcohols:
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv1p0025
Methyl mercaptan is too volatile (bp 6°C), the disulfide must be used, a procedure for generating it will come.
An example of the Cu-mercaptan coupling with aryl bromide:
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv5p0107
Related post (unsorted)
Post 360999
(Rhodium: "2,5-Dihydroxythiophenol and alkoxy derivatives", Novel Discourse) and pihkal #39
Post 360747
(Rhodium: "2C-T-x Precursors: Ar-I + R-SH -> Ar-S-R", Novel Discourse)Post 259352
(Rhodium: "Re: thiovanillin", Novel Discourse)Post 217091 (missing)
(poix: "Re: Preparing alkylthio Benzaldehydes 2CT2, 2CT7", Chemistry Discourse)Post 216802 (missing)
(foxy2: "Preparing alkylthio Benzaldehydes 2CT2, 2CT7 ect", Chemistry Discourse)Post 217440 (missing)
(poix: "Re: Preparing alkylthio Benzaldehydes 2CT2, 2CT7", Chemistry Discourse)Post 423119
(Chimimanie: "Synthesis of 2-Chloromethyl-5-alkylthio-DMB", Methods Discourse)Post 421991
(GC_MS: "2,5-dimethoxy-4-methylthiobenzaldehyde", Novel Discourse)Post 122786
(dormouse: "vanillin as a starting material for 2-CT's -rev drone", Serious Chemistry) w/ refs
https://www.thevespiary.org/rhodium/Rhodium/chemistry/4-alkylthio-25-dmb.html
Post 435958 (missing)
(Chimimanie: "Our old friend Poix", Chemistry Discourse)Post 414337
(Chimimanie: "This describe a grignard on ...", Novel Discourse)https://www.thevespiary.org/rhodium/Rhodium/chemistry/thiophenol.thiourea.html
https://www.thevespiary.org/rhodium/Rhodium/chemistry/bromodimethoxybenzaldehyde.html
Post 450928
(otto: "2,5-DMBA directly from 1,4-DMB", Novel Discourse)Post 454518
(Rhodium: "Two Syntheses of 2,5-Dimethoxybenzaldehyde", Chemistry Discourse)Post 448501
(Rhodium: "Aromatic Thiols by Electrophilic Substitution", Chemistry Discourse)Post 453098 (missing)
(azole: "áðîìèðîâàíèå 2,5-DMBA è äàëüøå â 2C-T-2", Russian HyperLab)To Bee Continued....
