These three papers deal with the addition of the O
2N-CH
2* radical on the aromatic ring yielding compounds of the type Ar-CH
2NO
2. The second paper was already referenced and the experimental part reported in the nice idea described in
Post 461187
(psyloxy: "2,5-diMeO-benzaldehyde,an unusual synth from 14DMB", Chemistry Discourse). Read that post and you will understand what this reaction is good for. But besides that it is also interesting in the view of its similarity to the radical acetonylation described in
https://www.thevespiary.org/rhodium/Rhodium/chemistry/p2p.manganese.html
.
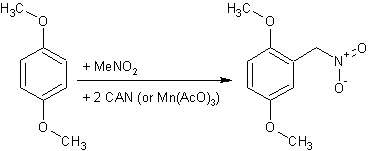 Nitromethylation of Aromatics with Nitromethane-Manganese(III) Acetate
Nitromethylation of Aromatics with Nitromethane-Manganese(III) AcetateMichael E. Kurz and Tsu-yu R. Chen
J. Org. Chem., 43(2) (
1978) 239-242.

Abstract: Manganese(III) acetate was found to effect aromatic substitution by a nitromethyl group when reacted with an aromatic and nitromethane in acetic acid. Based on similarities to previously studied maganese(III) acetate systems, a mechanism involving the generation of and substitution by nitromethyl radicals is proposed. The partial rate factors, p value (vs. p+) of -1.1, and the failure to substitute on nitrobenzene suggest that the nitromethyl radical exhibits appreciable electrophilic character. This reactioii, which proceeds in a clean, reasonably high-yield manner, might well provide an alternate route to synthesizing cerlain aryl nitromethanes, which are currently made through multistep, side-chain substitutions involving alpha-halo- or alpha-cyanotoluenes.Nitroalkylation of Aromatic Hydrocarbons Promoted by Manganese(III) AcetateMichael E. Kurz, Preecha Ngoviwatchai, and Tosaporn Tantrarant
J. Org. Chem. 46 (
1981) 4668-4672.

Abstract: Additional mechanistic and synthetic details concerning the formation of nitroalkylated aromatic products from the reaction of nitroalkanes with manganese(III) acetate and an aromatic compound are presented. A large isotope effect (ah/^d = 4.02-4.20) was found for both manganese(III)- and cerium(IV)-promoted nitromethylations with nitromethane-da while no isotope effect (ah/^d = 1-05) was observed with benzene-dg. This indicated that deprotonation (most likely from an aci radical cation) to the nitromethyl radical is the slow step while subsequent rearomatization of a o-radical complex occurs rapidly. Somewhat more convenient methods of activating the manganese(III) acetate promoter were found. However, attempts to find cooxidants or other additives which could improve this process met with only limited success. The reaction of either toluene or benzene with nitroethane or the nitropropanes and manganese(III) acetate in refluxing acetic acid gave much poorer yields (3-8%) of the corresponding nitroalkylated products. Incorporation of sodium acetate into these systems completely eliminated the aromatic substitution products and formed a-nitroalkyl acetates instead. Implications of this finding on the mechanism for the generation of nitroalkyl radicals are discussed.Free-Radical Aromatic Nitromethylation Promoted by Cerium(IV)Michael E. Kurz and Preecha Ngoviwatchai
J. Org. Chem. 46 (
1981() 4672-4676.

Abstract: A search for metal salt oxidants, other than manganese(III) acetate, capable of effecting aromatic nitromethylation with nitromethane was undertaken. Of the many screened, cerium(IV) salts showed the most promise. Cerium(IV) ammonium nitrate gave high, though initially erratic, yields (essentially 100% yield of isomeric a-nitroxylenes from toluene and a-nitrotoluene from benzene). However, nitration products and aromatic aldehydes were generated as well. Product formation was followed as a function of time in these reactions, and nitromethylation products were shown to be converted to the corresponding isomeric tolualdehydes or benzaldehyde upon prolonged heating. Cerium(III) nitrate was shown to be primarily responsible for promoting this side reaction. Cerium(IV) acetate, generated in solution by ozonolysis of either cerium(III) nitrate or a mixture of cerium(UI) acetate and cerium(III) nitrate in acetic acid, also was shown to promote high yields of nitromethylation products. Furthermore, this reaction was free of side products. Isomer studies and relative rates for nitromethylation were determined for both cerium(IV) salts in comparison with manganese(III) acetate. A Hammett treatment in each case gave similar p values (vs. p+): -2.3 from manganese(III) acetate, -2.0 from cerium(IV) ammonium nitrate, and -1.9 from cerium(IV) acetate, indicating a common intermediate in each case. The nitromethyl radical, believed to the substituting entity, exhibits marked electrophilic properties.
This one adds a 1,3-dicarbonyl compound (dimethyl malonate) to some benzene derivatives yielding Ar-CH(COOMe)
2. Even though these compounds can bee easily hydrolyzed/decarboxylated to arylacetic acids and these converted to appropriate PEA’s it is more interesting as a possibility to form various P2P’s if using ethyl acetoacetate or acetylacetone in place of dimethyl malonate (R=Me- and/or EtO-). Note that this radical reactions are performed in MeOH at room temperature as opposed to the acetonylation with Mn
3+/acetone since the malonate forms a radical way easier than acetone.
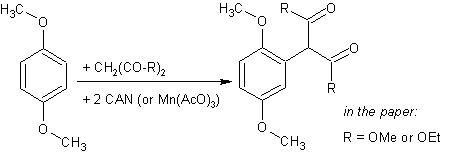 Dimethyl arylmalonates from cerium(IV) ammonium nitrate promoted reactions of dimethyl malonate with aromatic compounds in methanol
Dimethyl arylmalonates from cerium(IV) ammonium nitrate promoted reactions of dimethyl malonate with aromatic compounds in methanolEnrico Baciocchi, Donatella Dell'Aira, and Renzo Ruzziconi
Tetrahedron Letters, 27(24) (
1986) 2763-2766.

Summary: Aromatic compounds undergo homolytic malonylation by reaction with cerium(IV) ammonium nitrate and dimethyl malonate in methanol at room tenperature.
The drawback of these and similar radical reactions is the need of using very large excesses of the aromatic substrate and the need of preparing Mn
3+ or Ce
4+ salts (though CAN is commercially available). Luckily the aromatic substrates can bee recycled by various separation methods and the remaining metal salt reoxidized and both reused.