Trichlorosilane-Dimethylformamide (Cl3SiH-DMF) as an Efficient Reducing Agent.
Reduction of Aldehydes and Imines and Reductive Amination of Aldehydes under Mild Conditions Using Hypervalent HydridosilicatesShu Kobayashi, Masaru Yasuda, and lwao HachiyaChemistry Letters 407-408 (1996)Trichlorosilane-dimethylformamide (Cl
3SiH-DMF) was found to be an effective reducing agent for reduction of aldehydes to alcohols, imines to amines, and also reductive amination of aldehydes. Hypervalent silicates are active species, which enable efficient reduction under mild conditions.
While many reducing agents have been developed, trichlorosilane is among the most potentially promising reagent because it is cheap, stable, and easy to handle.
1 However, its use for the reduction of carbonyl compounds and imines is rather limited. Benkeser et al. reported that reductive silylation of carbonyl compounds proceeded using trichlorosilane-tertiary amine, and that the products in this reaction were not alcohols but alkylsilanes.
2 They also reported reduction of imines to amines under acetonitrile reflux conditions.
3 Sakurai and Kira reported that bis(1,2-benzendiolato)hydridosilicate, prepared from trichlorosilane and dilithium catecholate, reduced aldehydes and ketones to afford the corresponding alcohols.
4 In this paper, we report an efficient reducing agent, trichlorosilane-dimethylformamide (Cl
3SiH-DMF), which is effective for reduction of aldehydes to alcohols, imines to amines, and also reductive amination of aldehydes under mild conditions.
Recently, we have reported that some organosilicon reagents which are stable under normal conditions work as efficient nucleophiles in DMF.
5,6 For example, allyltrichlorosilanes,
5 propargylsilanes,
6 and allenylsilanes
6 react with carbonyl compounds in DMF without any catalysts (Lewis acids, fluoride anion, etc.) to afford the corresponding adducts such as homoallyl alcohols, homopropargylic and allenic alcohols, in high yields. In these reactions, DMF coordinates the silicon reagents to form active hypervalent organosilicate intermediates, which are sufficiently nucleophilic to carbonyl compounds.
Although hypervalent silicate reagents have been reported and some of them are used in organic synthesis,
7 ligands of most reagents are covalently bonded to silicon atoms. On the other hand, one of features of the reagents we developed is that ligands are coordinately bonded to silicon atoms, and hence they are easy to prepare and reactions can be performed under very mild conditions (neutral, 0°C to room temperature) . In the course of our investigations to develop new synthetic reactions using the reactive organosilicon reagents, we focused on Cl
3SiH-DMF as a hydride source and planned to develop a new reducing agent.
We first examined reduction of aldehydes, and it was found that Cl
3SiH worked most effectively as a reducing reagent in a DMF-dichloromethane mixed solvent system. We tested various aldehydes and selected examples are listed in Table 1. The reduction of aldehydes proceeded smoothly at 0°C for 4-6 h using Cl
3SiH in DMF-dichloromethane to give the corresponding alcohols in high yields.
8 Alkyltrichlorosilanes were not produced under these conditions.
2 No reaction occurred in dichloromethane, acetonitrile, benzene, and tetrahydrofuran (THF) even at RT (without DMF). Highly chemoselective reduction of aldehydes was achieved in the coexistence of an aryl chloride, aryl nitro group, and double and triple bonds. In the reduction of cinnamaldehyde, 1,2-addition of hydride was the main reaction (88%) and a 6% yield of 3-phenylpropanol was obtained. Reduction of 2-furyl and 2-thiophene aldehydes also worked well to afford the corresponding alcohols in high yields.
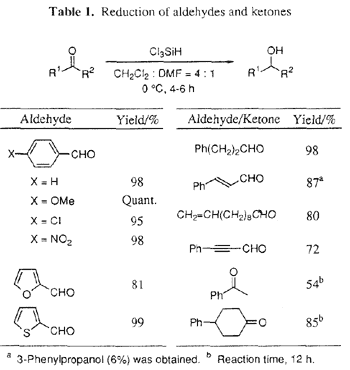
As for the reduction of ketones, a longer reaction time was required, and the secondary alcohols were obtained in good to high yields. Selective reduction of an aldehyde rather than a ketone was performed in more than 98% selectivity (Eq. 1).
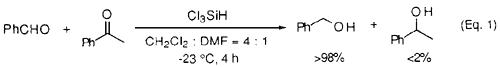
We then examined reduction of imines by using Cl
3SiH-DMF as a reductant. It was found that the reduction of imines also proceeded at 0°C in DMF-dichloromethane to give the corresponding secondary amines (Table 2). Moreover, reductive amination of aldehydes with amines was successfully carried out using Cl
3SiH-DMF (Table 3).
9 It is noted that aldehydes having alpha-protons, whose imines are known to be difficult to prepare, can be used in the reductive amination, and that the corresponding amines are obtained in high yields.
10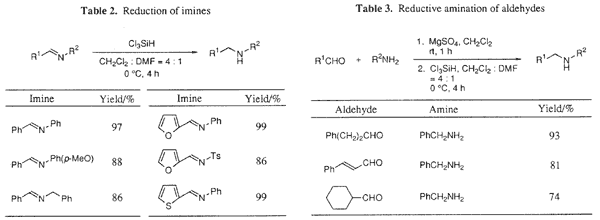
As for an active species in these reductions, the
29Si NMR spectra of Cl
3SiH-DMF in dichloromethane-d2 showed an equilibrium including hypervalent silicates. Signals were observed at -35.0, -181.8, -185.2, and -192.6 ppm (intensity; ca. 3:1:3:6),
11 and the latter three signals were assigned to six-coordinated silicons.
12 After adding 3-phenylpropanal in this system, the three signals at -181.8, -185.2, and -192.6 decreased and a new signal, which corresponds to a silicon of a reducing product, appeared at -184.6 ppm.
13 These results indicate that the active reducing species in these reductions should be hypervalent hydridosilicates.
ln summary, we have developed a new reducing agent, Cl
3SiH-DMF, which is effective for the reduction of aldehydes to alcohols, imines to amines, and also reductive amination of aldehydes under mild conditions. The reductions proceed smoothly without any catalyst (F-, etc.). Hypervalent hydridosilicates which are generated by coordination of DMF to Cl
3SiH are key species in this reduction, and it is noteworthy that the hydridosilicates can be prepared by simply mixing Cl
3SiH and DMF.
References and Notes[1] M. Hudlicky, "Reduction in Organic Chemistry," Ellis Horwood Limited, Chichester (1984).
[2] R. A. Benkeser and W. E. Smith, J. Am. Chem. Soc., 91, 1556 (1969).
[3] R. A. Benkeser and D. C. Snyder, J. Organmet. Chem., 225, 107 (1982).
[4] M. Kira, K. Sato, and H. Sakurai, J. Org. Chem., 52, 949 (1987).
[5] S. Kobayashi and K. Nishio, J. Org. Chem., 59, 6620 (1994).
[6] S. Kobayashi and K. Nishio. J. Am. Chem. Soc., 117, 6392 (1995).
[7] For example, M, Kira, K. Sato, and H. Sakurai, J. Am. Chem. Soc., 110, 4599 (1988); A. Hosomi, S. Kohra, K. Ogata, T. Yanagi, and Y. Tominaga, J. Org. Chem., 55, 2415 (1990); M. Fujita and T. Hiyama, J. Org. Chem., 53, 5405 (1988); G. Cerveau, C. Chuit, R. J. P. Cornu, and C. Reye, J. Organomet. Chem., 328, C17 (1987); M. Kira, M. Kobayashi, and H. Sakurai, Tetrahedron Lett., 28, 4081 (1987).
[8] A typical experimental procedure for the reduction of an aldehyde: To an aldehyde (0.4 mmol) in DMF-CH2Cl2 (1:3, 3 ml) was added Cl3SiH (0.6 mmol) in CH2Cl2 (1 ml) at 0°C. The mixture was stirred for 4-6 h at this temperature, and MeOH (1 ml) was added. Water was then added and insoluble materials were removed by filtration. The organic layer was separated and the aqueous layer was extracted with CH2Cl2. After a usual work up, the corresponding alcohols were obtained.[9] For reductive amination using borohydrides, A. F. Abdel-Magid, C. A. Maryanoff, and K. G. Carson, Tetrahedron Lett., 31, 5595 (1990); R. F. Borch, M. D. Bernstein, and H. D. Durst, J. Am. Chem. Soc., 93, 2897 (1971).
[10] A typical experimental procedure for the reductive amination of an aldehyde: An aldehyde (0.4 mmol), an amine (0.4 mmol), and MgSO4 (125 mg) were combined in CH2Cl2 (1.5 ml) at rt for 1 h. After cooling to 0°C, Cl3SiH (0.6 mmol) in DMF-CH2Cl2 (1:3, 3 ml) was added. The mixture was stirred for 4 h at this temperature, and MeOH (1 ml) was then added. Saturated aqueous sodium bicarbonate was then added and insoluble materials were removed by filtration. After 1 M NaOH solution was added, the organic layer was separated and the aqueous layer was extracted with CH2Cl2. After a usual work up, the corresponding amines were obtained. [11] Measured at 20 °C (0.25 M).
[12] H. Marsmann, in "NMR, Oxygen-17 and Silicon-29," Ed. by P. Diehl, E. Ruck, and R. Kosfeld, Springer-Verlag, Berlin (1981), Vol. 17, p. 65; J. A. Cella, J. D. Cargiolo, and E. A. Williams, J. Organomet. Chem., 186, 13 (1980).
[13] The signal at -184.6 ppm was confirmed by comparison with that of the authentic sample prepared from 3-phenylpropanol, triethylamine, and tetrachlorosilane.